Baby gassy foods
What You Can Do To Help Your Gassy Breastfed Baby
What You Can Do To Help Your Gassy Breastfed Baby | MedelaWe use essential and functional cookies, which are necessary to display our website correctly, and additional cookies to provide you with more personalized information and marketing. Additionally, we use anonymized analytics cookies to review our traffic and to allow the best experience possible whenever you visit. We also share the anonymized information about your use of our site with analytics partners. For more information please visit our Human Milk Website Privacy & Cookie Policy.
Cart
Added to your cart!
Checkout
View Cart
Buy/Rent A Pump Sign InBreast Pump Rental Free Breast Pump Join Medela Family
All Products Breast Pumps Storage Feeding Accessories Spare Parts Cleaning Apparel Breast Care
Breast Pump Rental Breastfeeding Guide Ask the LC NICU Feeding Product Help
- Medela US
- Breastfeeding Education, Tools, and Support
- Breastfeeding Guide
- Signs and Solutions For Gassy Breastfed Babies
What are some common culprits behind your baby's gassiness? Learn the signs, foods that may cause gassiness in your baby, and how to soothe and relieve his or her symptoms.
Share this content
As a new parent, it can be stressful and upsetting to see and hear your baby cry. That’s especially true if you've checked off all the usual suspects— dirty diaper, empty belly, discomfort, or over-tiredness —and you still can’t seem to soothe your little one.
Gas is something that many newborns experience, and it can be painful for them! It isn't always the first thing that parents remember to consider, since it's not something easily visible.
Signs Your Breastfed Baby is Gassy
If you suspect excess gas could be the culprit causing your baby’s fussiness, there are several signs that may indicate you are correct:
- Burping. It’s possible your baby has swallowed too much air while nursing or crying for a long period.
- Spitting up. While spitting up is perfectly normal, gas that’s trapped in the stomach can push breast milk back up and cause your baby to spit up.
- Bloated tummy. This could be a sign that gas has built up in your baby’s stomach.
- Flatulence. Every baby toots, but if they’re doing so excessively, it could mean they have excess gas.
- Arched back, legs drawn toward the tummy. The discomfort from gas pains will make a baby try to adjust to alleviate it.
Gassy Baby Causes
Gas in a breastfed baby is not uncommon and can be attributed to several factors:
- Gulping while feeding.
 If your milk let-down reflex is strong, your baby may gulp your milk to keep up and swallow extra air in the process. If that’s the case, your little one may do better nursing in a more upright position, so he or she has better control over milk intake and flow.
If your milk let-down reflex is strong, your baby may gulp your milk to keep up and swallow extra air in the process. If that’s the case, your little one may do better nursing in a more upright position, so he or she has better control over milk intake and flow. - Introducing a bottle. If your baby is used to the breast and you begin feeding with a bottle, it may take some getting used to at first. As a result, he or she may swallow too much air while eating.
- Constipation. When your baby is constipated, they may have gas trapped in their tummies that they’re having a hard time releasing.
- Crying. If your baby has been crying for a long time, they may be gulping in air in the process.
- Mom’s diet. Food that you’ve eaten can make your baby gassy as well. Certain foods such as dairy, soy or wheat may contribute to gassiness in your little one. Keep a food journal of what you eat to see if you can pinpoint the culprit in your diet.

Foods That Make Breastfed Babies Gassy
Though a baby’s gas is not commonly linked to mom’s diet, there are certain gas-inducing foods that could give both a breastfeeding mom and her baby gas. These include:
- Fiber. Foods like bran, beans, and whole grains.
- Fruit. Citrus fruits, prunes, plums, peaches, or apricots.
- Vegetables. Broccoli, cabbage, and Brussel sprouts.
- Garlic. Garlic-seasoned foods like pasta dishes or garlic bread.
- Dairy. Yogurt, ice cream, or milk products.
- Carbonated beverages. If they make you burp, they could make your baby gassy too.
It’s not necessary to give up all your favorite foods when pregnant and/or breastfeeding. Health experts recommend only making dietary changes if you see a direct connection between something you’ve eaten and your baby's gassiness.
Additionally, if you’re still breastfeeding after your little one begins solids or finger foods, it’s easier to detect what food might be the culprit and then eliminate it.
Relieving Gassy Babies
There are several effective ways to help relieve your baby’s gas pains and soothe them. Try a combination of these to find what works best for your little one.
- Burp twice. Try to coax two burps out of your baby instead of just one.
- Sit upright. Hold your baby in an upright position while burping. This makes it easier to expel gas.
- Tummy time. Laying your baby on their tummy will help to push gas out.
- Bicycle exercises. Put your baby on his or her back and move their legs in a pedaling motion, similar to cycling on a bike. This helps with constipation as well.
- Massage the tummy. A gentle massage can help move gas out.
- Adjust baby’s latch. Make sure your baby is latching correctly to avoid swallowing too much air.
Don't worry, mama - Gas is typically a normal occurrence and most babies experience gassiness from time to time! With some minor adjustments, you can soothe your little one and help them get through the discomfort of gas.
How to Help Baby or Toddler With Gas – Foods to Avoid
Dr. Deena Blanchard
All people pass gas! In fact, it is normal to pass gas anywhere from six to twenty times a day. Additional gassiness is typically not related to a serious health problem and will often resolve without any intervention. When gas becomes painful or bothersome for your little one, it is important to get in touch with your pediatrician.
Gas when Starting Solids
As your little one gets older and you start to introduce solid foods, you may find that certain foods increase gassiness. It is important to differentiate between gas that is painful and gas that is not. If your little one is passing gas frequently but seems happy and playful and without abdominal pain then there is no reason to intervene or make changes.
Foods that break down more slowly, such as beans, cruciferous vegetables (broccoli, cauliflower, kale, etc..), and other high fiber containing foods can cause additional gas. These foods are rich in nutrients and fiber and an important part of your little one’s diet. It may take some time for your little one’s digestive system to adjust to these foods and that is okay. If your baby is in pain, vomiting, irritable or has blood in the stool you should always call your pediatrician.
These foods are rich in nutrients and fiber and an important part of your little one’s diet. It may take some time for your little one’s digestive system to adjust to these foods and that is okay. If your baby is in pain, vomiting, irritable or has blood in the stool you should always call your pediatrician.
Making sure your little one is well hydrated as you introduce solid foods and particularly high fiber foods, can minimize gassiness and prevent constipation.
Gas in the Older Infant and Toddler Diet
Common culprits for increasing gas in older children are:
- Fried and fatty foods.
- Beans.
- Vegetables such as artichokes, asparagus, broccoli, brussels sprouts, cabbage, cauliflower, cucumbers, green peppers, onions, peas, radishes, and raw potatoes.
- Fruits such as apricots, bananas, melons, peaches, pears, prunes, and raw apples.
- Wheat and wheat bran.
Keeping a food and symptom log can help determine if specific foods are increasing your child’s symptoms. Symptoms of gas can include, passing gas, bloating, mild abdominal discomfort, and belching. If you are finding certain foods are causing your child to have these symptoms or feel uncomfortable, it is best to discuss this with their pediatrician.
Symptoms of gas can include, passing gas, bloating, mild abdominal discomfort, and belching. If you are finding certain foods are causing your child to have these symptoms or feel uncomfortable, it is best to discuss this with their pediatrician.
Drinking lots of water and getting regular exercise and movement can help decrease symptoms of gas and bloating. Avoiding carbonated beverages and drinks containing sorbitol or xylitol is another helpful way to prevent gas build up. As always, if you have any concerns and before making dietary changes it is best to talk to your pediatrician.
Sources
American Gastroenterological Association http://www.gastro.org/wmspage.cfm?parm1=689
The American College of Gastroenterology http://www.acg.gi.org/patients/gihealth/belching.asp
American Academy of Pediatrics: “Abdominal Pain.”
American Academy of Family Physicians: “Feeding Problems in Infants and Children.”
Published: February 10, 2020
Last modified: February 10, 2020
Products for eliminating or preventing gas in the stomach
There is nothing more irritating than gas, and it is quite common not only in adults but also in children. Swallowing large amounts of air during meals, together with the action of intestinal bacteria, causes the formation of irritating and unpleasant gases in the intestinal tract.
Swallowing large amounts of air during meals, together with the action of intestinal bacteria, causes the formation of irritating and unpleasant gases in the intestinal tract.
This is why you should avoid certain foods in your children's diet, since consuming many of them is usually the reason why kids have a bad time. In contrast, there are other foods that can help prevent and counteract gas in the stomach.
Index
- 1 Causes and symptoms of gas
- 2 Foods to help avoid gas
- 3 A few tips to prevent gas in children
Causes and symptoms of gas
Gas in the stomach usually occurs for two reasons: 3 Aerophagia when too much air is swallowed while eating or due to breathing problems. In the case of babies, it is important that they can suckle both from the mother's breast and from the bottle. As for children, it is important to monitor their diet so that they do not have irritating gas.
With regard to irritating gases, the child may have several symptoms, such as excessive bloating, pain in the intestinal area, flatulence in both the rectum and mouth in the form of belching. The truth is that gas is very annoying and many children have a hard time with this stomach problem.
Foods to Avoid Gas
As we mentioned above, food is key when it comes to keeping the little ones in the house free of those damn gases. Therefore, nutritionists advise to include foods such as:
- Fruit, such as pineapple or papaya.
- Yogurt with bifidus as they contain living organisms that help balance the intestinal flora.
- Chicken or turkey meat and fish These are high quality protein foods that are well digested in the intestines.
- Infusions can also be very good at preventing gas in children.
 although it is always recommended to consult your pediatrician before choosing their .
although it is always recommended to consult your pediatrician before choosing their .
On the contrary, there are a number of foods that can cause gas, so it is necessary to reduce their intake in children's diets:
- Legumes are rather gaseous foods that cause gas in the stomach. That's why you should reduce your intake of beans or peas.
- Vegetables such as Brussels sprouts or cauliflower. they are also not recommended for those children who are very prone to gas.
- Carbonated drinks are also not recommended when it comes to cutting out gas.
Some tips for preventing gas in children
- Experts advise moving after eating. Lying down immediately after eating usually causes gas.
- For lunch, it is advisable to choose boiled, cooked or grilled foods and forget about fried foods.

- Eating slowly and with your mouth closed is the key to preventing too much air from entering your own mouth. Haste is bad advice, so it is always recommended to chew food calmly and enjoy it.
- When it comes to drinking, it's best to drink in a glass and avoid the famous straws. In the case of infants, it is good to buy an anti-colic bottle and thus avoid the formation of gases.
- Do not let your child chew gum regularly Because when more air than usual enters the mouth, the above gases are formed.
The content of the article complies with our principles of editorial ethics. To report a bug, click here.
You may be interested
Combustion (combustion) products: composition, substances, classification
Combustion products are substances (gaseous, liquid or solid substances) and compounds formed as a result of a complex physical and chemical combustion process of substances (materials).
Combustion products are most often understood as smoke, toxic combustion products, soot and others.
Combustion products of dry grass
Knowledge of the properties and quantity of combustion products is necessary to calculate the heat of combustion, combustion temperature and other indicators used to assess the fire and explosion hazard of substances (materials), objects with the presence of these substances (materials).
Composition
Their composition depends on the composition of the burning substance and the conditions of its combustion. Under fire conditions, organic substances most often burn (wood, fabrics, gasoline, kerosene, rubber, etc.), which mainly include carbon, hydrogen, oxygen and nitrogen. When they are burned in a sufficient amount of air and at a high temperature, products of complete combustion are formed: CO 2 , H 2 O, N 2 . When burning in an insufficient amount of air or at a low temperature, in addition to the products of complete combustion, products of incomplete combustion are formed: CO, C (soot).
Combustion products are called wet if the water vapor content is taken into account when calculating their composition, and dry if the water vapor content is not included in the calculation formulas.
Less commonly, inorganic substances, such as sulfur, phosphorus, sodium, potassium, calcium, aluminum, titanium, magnesium, etc., burn during a fire. In most cases, their combustion products are solid substances, for example P 2 O 5 , Na 2 O 2 , CaO, MgO. They are formed in a dispersed state, so they rise into the air in the form of dense smoke. The products of combustion of aluminum, titanium and other metals in the combustion process are in a molten state.
During incomplete combustion of organic substances at low temperatures and lack of air, more diverse products are formed - carbon monoxide, alcohols, ketones, aldehydes, acids and other complex chemical compounds. They are obtained by partial oxidation of both the fuel itself and the products of its dry distillation (pyrolysis). These products produce acrid and poisonous smoke. In addition, the products of incomplete combustion themselves are capable of burning and forming explosive mixtures with air. Such explosions occur when extinguishing fires in basements, dryers and in enclosed spaces with a large amount of combustible material. Let us briefly consider the properties of the main combustion products.
These products produce acrid and poisonous smoke. In addition, the products of incomplete combustion themselves are capable of burning and forming explosive mixtures with air. Such explosions occur when extinguishing fires in basements, dryers and in enclosed spaces with a large amount of combustible material. Let us briefly consider the properties of the main combustion products.
Carbon dioxide
Carbon dioxide or carbon dioxide (CO 2 ) is a product of complete combustion of carbon. Has no smell and color. Its density relative to air is 1.52. The density of carbon dioxide at a temperature of T \u003d 0 ° C and at normal pressure p \u003d 760 millimeters of mercury (mm Hg) is 1.96 kg / m 3 (air density under the same conditions is ρ \u003d 1, 29 kg/m 3 ). Carbon dioxide is highly soluble in water (at T = 15 °C one liter of gas dissolves in one liter of water). Carbon dioxide does not support combustion of substances, with the exception of alkali and alkaline earth metals. The combustion of magnesium, for example, occurs in an atmosphere of carbon dioxide according to the equation:
The combustion of magnesium, for example, occurs in an atmosphere of carbon dioxide according to the equation:
CO 2 +2 Mg \u003d C + 2 MgO .
Carbon dioxide toxicity is negligible. The concentration of carbon dioxide in the air of 1.5% is harmless to humans for a long time. When the concentration of carbon dioxide in the air exceeds 3-4.5%, being indoors and inhaling the gas for half an hour is life-threatening. At a temperature of T = 0 °C and pressure p = 3.6 MPa carbon dioxide turns into a liquid state. The boiling point of liquid carbon dioxide is T = -78 °C. With the rapid evaporation of liquid carbon dioxide, the gas cools and turns into a solid state. Both in liquid and solid state, drops and powders of carbon dioxide are used to extinguish fires.
Carbon monoxide
Carbon monoxide or carbon monoxide (CO) is a product of incomplete combustion of carbon. This gas is odorless and colorless, therefore it is especially dangerous. Relative density is 0.97. The density of carbon monoxide at T \u003d 0 ° C and p \u003d 760 mm Hg is 1.25 kg / m 3 . This gas is lighter than air and accumulates in the upper part of the room during fires. Carbon monoxide is almost insoluble in water. It is capable of burning and forms explosive mixtures with air. Carbon monoxide, when burned, produces a blue flame. Carbon monoxide is highly toxic. Inhalation of air with a carbon monoxide concentration of 0.4% is fatal to humans. Standard gas masks do not protect against carbon monoxide, so special filters or oxygen isolating devices are used in case of fires.
Relative density is 0.97. The density of carbon monoxide at T \u003d 0 ° C and p \u003d 760 mm Hg is 1.25 kg / m 3 . This gas is lighter than air and accumulates in the upper part of the room during fires. Carbon monoxide is almost insoluble in water. It is capable of burning and forms explosive mixtures with air. Carbon monoxide, when burned, produces a blue flame. Carbon monoxide is highly toxic. Inhalation of air with a carbon monoxide concentration of 0.4% is fatal to humans. Standard gas masks do not protect against carbon monoxide, so special filters or oxygen isolating devices are used in case of fires.
Water
Well-known water – H 2 O – also released during combustion as a gas – as steam. Water is a combustion product of methane gas - CH 4 . In general, water and carbon dioxide are mainly released during the complete combustion of all organic substances.
Hydrogen cyanide
Potassium cyanide - the strongest poison - the salt of hydrocyanic acid, also known as hydrogen cyanide - HCN. It is a colorless liquid, but very volatile (easily turning into a gaseous state). That is, during combustion, it will also be released into the atmosphere in the form of gas. Hydrocyanic acid is very toxic, even a small concentration in the air - 0.01 percent - is fatal. A distinctive feature of the acid is the characteristic smell of bitter almonds. But hydrocyanic acid has one “highlight” - it can be poisoned not only by inhaling directly with the respiratory system, but also through the skin. So it will not work to protect yourself only with personal protective equipment for the respiratory system and vision.
It is a colorless liquid, but very volatile (easily turning into a gaseous state). That is, during combustion, it will also be released into the atmosphere in the form of gas. Hydrocyanic acid is very toxic, even a small concentration in the air - 0.01 percent - is fatal. A distinctive feature of the acid is the characteristic smell of bitter almonds. But hydrocyanic acid has one “highlight” - it can be poisoned not only by inhaling directly with the respiratory system, but also through the skin. So it will not work to protect yourself only with personal protective equipment for the respiratory system and vision.
Acrolein
Propenal, acrolein, acrylaldehyde - all these are the names of one substance, unsaturated acrylic acid aldehyde: CH 2 \u003d CH-CHO. This aldehyde is also a highly volatile liquid. Acrolein is colorless, with a pungent odor, and is very toxic. If liquid or its vapors come into contact with mucous membranes, especially in the eyes, it causes severe irritation. Propenal is a highly reactive compound, and this explains its high toxicity.
Propenal is a highly reactive compound, and this explains its high toxicity.
Formaldehyde
Like acrolein, formaldehyde belongs to the class of aldehydes and is an aldehyde of formic acid. This compound is also known as methanal. It is a toxic, colorless gas with a pungent odor.
Nitrogen-containing substances
Most often, during the combustion of substances containing nitrogen, pure nitrogen is released - N 2 . This gas is already present in the atmosphere in large quantities. Nitrogen can be an example of a combustion product of amines. But during thermal decomposition, for example, ammonium salts, and in some cases during combustion itself, its oxides are also emitted into the atmosphere, with the degree of nitrogen oxidation in them plus one, two, three, four, five. Oxides are gases that are brown in color and extremely toxic.
Sulfur dioxide
Sulfur dioxide (SO 2 ) is a combustion product of sulfur and sulfur compounds. A colorless gas with a characteristic pungent odor. The relative density of sulfur dioxide is 2.25. The density of this gas at T \u003d 0 ° C and p \u003d 760 mm Hg is 2.9 kg / m 3 , that is, it is much heavier than air. Sulfur dioxide dissolves well in water, for example, at a temperature of T \u003d 0 ° C, eighty liters of SO 9 dissolve in one liter of water0128 2 , and at T = 20 ° C - forty liters. Sulfur dioxide does not support combustion. It acts irritatingly on the mucous membranes of the respiratory tract, as a result of which it is very toxic.
A colorless gas with a characteristic pungent odor. The relative density of sulfur dioxide is 2.25. The density of this gas at T \u003d 0 ° C and p \u003d 760 mm Hg is 2.9 kg / m 3 , that is, it is much heavier than air. Sulfur dioxide dissolves well in water, for example, at a temperature of T \u003d 0 ° C, eighty liters of SO 9 dissolve in one liter of water0128 2 , and at T = 20 ° C - forty liters. Sulfur dioxide does not support combustion. It acts irritatingly on the mucous membranes of the respiratory tract, as a result of which it is very toxic.
Smoke
During the combustion of many substances, in addition to the products of combustion discussed above, smoke is released - a dispersed system consisting of the smallest solid particles suspended in a gas. The diameter of the smoke particles is between 10 −4 to 10 −6 cm (from 1 to 0.01 µm). Note that 1 micron (micron) is equal to 10 −6 m or 10 −4 cm. Larger solid particles formed during combustion quickly settle in the form of soot and soot. When burning organic substances, the smoke contains particulate soot suspended in CO 2 , CO, N 2 , SO 2 and other gases. Depending on the composition and combustion conditions of the substance, smokes of different composition and color are obtained. When burning wood, for example, grayish-black smoke is formed, fabrics - brown smoke, oil products - black smoke, phosphorus - white smoke, paper, straw - whitish-yellow smoke.
Larger solid particles formed during combustion quickly settle in the form of soot and soot. When burning organic substances, the smoke contains particulate soot suspended in CO 2 , CO, N 2 , SO 2 and other gases. Depending on the composition and combustion conditions of the substance, smokes of different composition and color are obtained. When burning wood, for example, grayish-black smoke is formed, fabrics - brown smoke, oil products - black smoke, phosphorus - white smoke, paper, straw - whitish-yellow smoke.
In addition to the products of complete and incomplete combustion, the composition of smoke generated from fires during the combustion of organic substances contains products of thermal-oxidative decomposition of combustible substances. They are formed by heating still non-burning combustible substances in the air or smoke containing oxygen. This usually occurs in front of the flame or in the upper parts of the premises where the heated combustion products are located.
The composition of the products of thermal-oxidative decomposition depends on the nature of combustible substances, temperature and conditions of contact with the oxidizing agent. Thus, studies show that during the thermal-oxidative decomposition of combustible substances, the molecules of which contain hydroxyl groups, water is always formed. If the combustible substances contain carbon, hydrogen, and oxygen, the products of thermal-oxidative decomposition are most often hydrocarbons, alcohols, aldehydes, ketones, and organic acids. If the composition of combustible substances, in addition to the listed elements, contains chlorine or nitrogen, then the smoke also contains hydrogen chloride and cyanide, nitrogen oxides and other compounds. Thus, the smoke during the combustion of capron contains hydrogen cyanide, during the combustion of linoleum "Relin" - hydrogen sulfide, sulfur dioxide, during the combustion of organic glass - nitrogen oxides. The products of incomplete combustion and thermal-oxidative decomposition in most cases are toxic substances, therefore, indoor fires are extinguished only in oxygen insulating gas masks.
Ashes, ash, soot, soot, coal
Soot, or soot - the remains of carbon that did not react, for various reasons. Soot is also called amphoteric carbon. Ash or ash Fine particles of inorganic salts that have not burned or decomposed at the combustion temperature. When the fuel burns out, these microcompounds pass into a suspended state or accumulate at the bottom. And coal is a product of incomplete combustion of wood, that is, its remains that have not burned down, but are still able to burn. Of course, these are far from all the compounds that are released during the combustion of certain substances. To list them all is unrealistic, and it is not necessary, because other substances are released in negligible amounts, and only when certain compounds are oxidized.
Classification
Most combustion products are toxic. Therefore, speaking about their classification, it would be correct to familiarize you with the following term:
Hazard classification of substances according to the degree of impact on the body is the establishment (ranking) of the hazard levels of substances according to their damaging and ) animal. Read more about this classification in the material at the link >>
Also read the educational material on the topic:
Toxicity of combustion products
Toxicity index of combustion products
Formulas for calculating the volume substances.
Individual chemical compound
In this case, the calculation is based on the combustion reaction equation. The volume of wet combustion products of a unit mass (kg) of a combustible substance under normal conditions is calculated by the formula:
where:
V p.s. – volume of wet combustion products, m 3 /kg; - the number of kilomoles of carbon dioxide, water vapor, nitrogen and combustible substance in the combustion reaction equation; M - the mass of the combustible substance, numerically equal to the molecular weight, kg.
For example, to determine the volume of dry products of combustion of 1 kg of acetone under normal conditions, we compose the reaction equation for the combustion of acetone in air:
CH 3 COCH 3 + 4OO 2 + 4 · 3. 76N 2 = 3CO 2 + 3H 2 O + 4 · 3.76N 2
76N 2 = 3CO 2 + 3H 2 O + 4 · 3.76N 2
combustion of acetone:
The volume of wet combustion products 1 m 3 combustible substance (gas) can be calculated by the formula:
where:
V p.s. - volume of wet combustion products 1 m 3 combustible gas, m 3 /m 3 ; - the number of moles of carbon dioxide, water vapor, nitrogen and combustible substance (gas).
A complex mixture of chemical compounds
If the elemental composition of a complex combustible substance is known, then the composition and amount of combustion products of 1 kg of the substance can be determined by the equation for the combustion reaction of individual elements. To do this, the equations for the reaction of combustion of carbon, hydrogen, sulfur are compiled and the volume of combustion products per 1 kg of combustible substance is determined. The combustion reaction equation has the form:
C + O 2 + 3. 76N 2 \u003d CO 2 + 3.76N 2
76N 2 \u003d CO 2 + 3.76N 2
When burning 1 kg of carbon, 22.4 10 9 2 and 22.4 × 3.76/12 = 7.0 m 3 N 2 .
Similarly determine the volume (in m 3 ) of combustion products of 1 kg of sulfur and hydrogen. The received data is shown below:
| CO 2 | N 2 | H 2 O | SO 2 | |
| Carbon | 1.86 | 7.00 | – | – |
| Hydrogen | – | 21.00 | 11.2 | – |
| Sulfur | – | 2.63 | – | 0.7 |
When carbon, hydrogen and sulfur are burned, oxygen comes from the air. However, the composition of the combustible substance may include oxygen, which also takes part in combustion. In this case, air is consumed correspondingly less for the combustion of the substance.
Combustibles may contain nitrogen and moisture, which are converted into combustion products during combustion. To account for them, it is necessary to know the volume of 1 kg of nitrogen and water vapor under normal conditions.
The volume of 1 kg of nitrogen is equal to 0.8 m 3 , and the volume of water vapor is 1.24 m 3 . In air at 0 ° C and a pressure of 101325 Pa, 1 kg of oxygen accounts for 3.76 × 22.4 / 32 = 2.63 m 3 nitrogen.
Based on the given data, the composition and volume of combustion products of 1 kg of combustible substance are determined.
For example, to determine the volume and composition of the wet products of combustion of 1 kg of coal, consisting of 75.8% C, 3.8% H, 2.8% O, 1.1% N, 2.5 % S, W = 3.8%, A = 11.0%.
The volume of combustion products will be as follows, m 3 :
| CO 2 | H 2 O | N 2 | SO 2 | |
| Carbon | 1. 86 × 0.758 = 1.4 86 × 0.758 = 1.4 | – | 7 × 0.758 = 5.306 | – |
| Hydrogen | – | 11.2 × 0.038 = 0.425 | 21 × 0.038 = 0.798 | – |
| Sulfur | – | – | 2.63 × 0.025 = 0.658 | 0.7 × 0.025 = 0.017 |
| Nitrogen in fuel | – | – | 0.8 x 0.011 = 0.0088 | – |
| Moisture in fuel | – | 1.24 × 0.03 = 0.037 | – | – |
| Amount | 1.4 | 0.462 | 6.7708 - 0.0736 = 6.6972 | 0.017 |
Subtract the amount of nitrogen attributable to oxygen in coal 0. 028 × 2.63 = 0.0736 m The result indicates the composition of the combustion products of coal: the volume of wet combustion products of 1 kg of coal is:
028 × 2.63 = 0.0736 m The result indicates the composition of the combustion products of coal: the volume of wet combustion products of 1 kg of coal is:
V p.s. = 1.4 + 0.462 + 6.6972 + 0.017 = 8.576 m 3 /kg.
Mixture of gases
The amount and composition of combustion products for a mixture of gases is determined by the equation of the combustion reaction of the components that make up the mixture. For example, methane burning proceeds according to the following equation:
SN 4 + 2O 2 + 2 × 3.76N 2 2 + 2N 2 O + 7.52N 2
according to this equation, when burning 1 m 3 methane is obtained 1 m 3 carbon dioxide, 2 m 3 water vapor and 7.52 m 3 nitrogen. Similarly, the volume (in m 3 ) of combustion products of 1 m 3 of various gases is determined:
| CO 2 | H 2 O | N 2 | SO 2 | |
| Hydrogen | – | 1.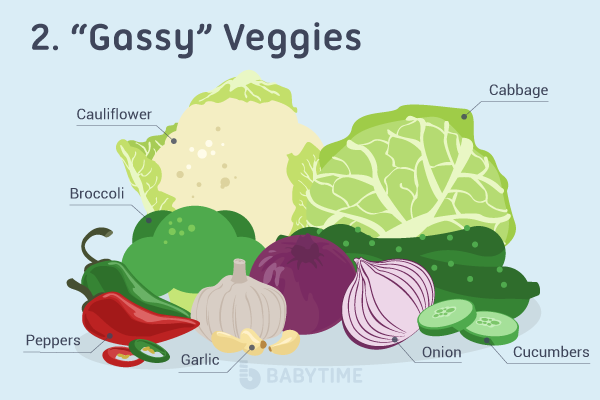 0 0 | 1.88 | - |
| Carbon monoxide | 1.0 | – | 1.88 | – |
| Hydrogen sulfide | – | 1.0 | 5.64 | 1.0 |
| Methane | 1.0 | 2.0 | 7.52 | – |
| Acetylene | 2.0 | 1.0 | 9.54 | – |
| Ethylene | 2.0 | 2.0 | 11.28 | – |
Based on the figures given, the composition and amount of combustion products of the gas mixture are determined.
Analysis of combustion products taken from fires in various rooms shows that they always contain a significant amount of oxygen. If a fire occurs in a room with closed window and door openings, then the fire in the presence of fuel can continue until the oxygen content in the mixture of air with combustion products in the room decreases to 14-16% (vol.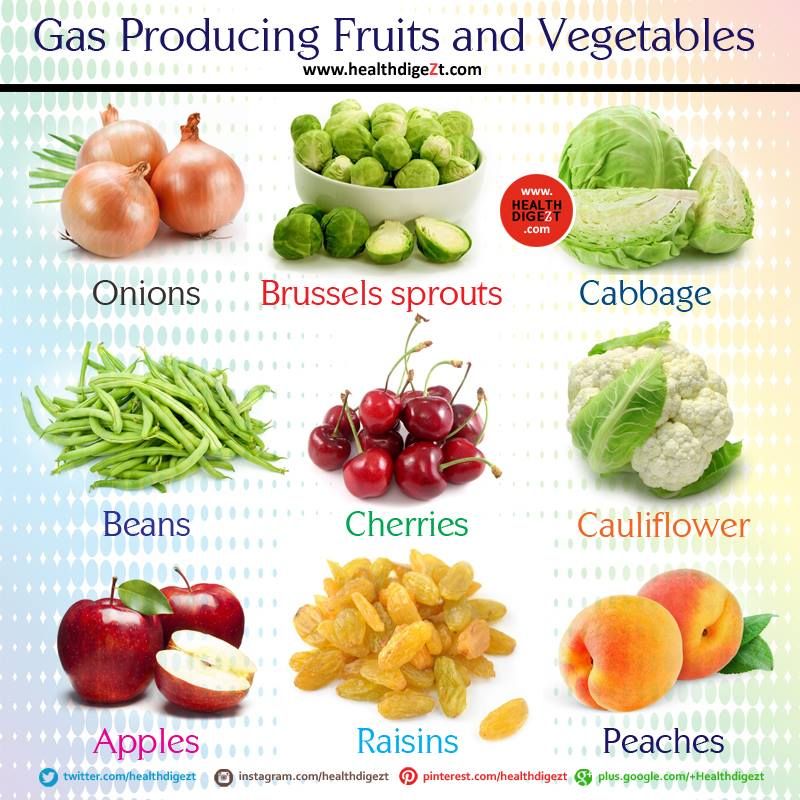 ). Consequently, on fires in enclosed spaces, the oxygen content in the combustion products can be in the range from 21 to 14% (vol.). The composition of combustion products during fires in rooms with open openings (basement, attic) shows that the oxygen content in them can be below 14% (vol.):
). Consequently, on fires in enclosed spaces, the oxygen content in the combustion products can be in the range from 21 to 14% (vol.). The composition of combustion products during fires in rooms with open openings (basement, attic) shows that the oxygen content in them can be below 14% (vol.):
| SO | CO 2 | O 2 | |
| In cellars | 0.15-0.5 | 0.8-8.5 | 10.6-19 |
| Attics | 0.1-0.6 | 0.3-4.0 | 16.0-20.2 |
According to the content of oxygen in the products of combustion on fires, one can judge the coefficient of excess air at which combustion occurred.
Effects on the human body
The degree of toxicity of substances is related to their physical and chemical nature. Interacting with the body, combustion products cause pathological syndromes.
International classification of diseases of the tenth revision ICD-10 defines poisoning by combustion products code T59 - "Toxic effect of other gases, fumes and vapors. "
"
According to the mechanism of action on humans, toxic components in smoke are divided into five groups.
- Substances that cause damage to the skin and mucous membranes. Symptoms of such poisoning by combustion products are itching, burning of the skin and its inflammation, pain in the eye area, eyelids, lacrimation, cough. Examples are tar fumes, sulfur dioxide, formaldehyde.
- Combustion products which cause acute inhalation poisoning. The victims complain of shortness of breath, cough. On examination, attention is drawn to frequent breathing, cyanosis. At high concentrations of toxic gas, respiratory arrest may occur. So, signs of poisoning with PVC combustion products can appear after a few hours. Inhalation poisoning causes chlorine, ammonia, nitric oxide.
- Combustion products with the formation of toxic substances, which are called "blood poisons". By binding hemoglobin, they disrupt the access of oxygen to tissues and trigger pathological reactions that cover the entire body.
 Examples are carbon monoxide, nitrogen dioxide.
Examples are carbon monoxide, nitrogen dioxide. - Combustion products for which the nervous system is the target organ. This is benzene, hydrogen sulfide.
- Enzyme poisons that affect tissue respiration by blocking oxygen activation processes. This is hydrogen sulfide, hydrocyanic acid.
Many toxins that form in combustion products are "universal", as they cause damage to several body systems at once.
First aid for poisoning
Symptoms of intoxication with different substances may vary, but the principles of first aid are always the same.
Most poisons are inhaled. The first thing to do in case of poisoning is to stop the intake of combustion products into the body. For this you need:
- observing safety and if there is such an opportunity to stop the flow of toxic substances - gas, smoke;
- ventilate the room or other volume where the victim is located;
- remove contaminated clothing;
- If there are no contraindications, move the casualty to a safe place.

Acute intoxication requires emergency treatment. Actions in case of poisoning by combustion products are as follows:
- call an ambulance;
- in case of smoke, provide ways to protect the respiratory organs from combustion products;
- if there are symptoms of irritation, rinse eyes, mouth, nose;
- in the absence of consciousness, give the victim a horizontal position and ensure the patency of the respiratory tract;
- before the arrival of medical specialists, observe consciousness, breathing, heart rate, blood pressure;
- if there are signs of a terminal condition, then proceed to cardiopulmonary resuscitation.
Some inhalation poisonings with combustion products have a period of apparent well-being. Even in the absence of pathological symptoms, it is worth carefully monitoring the condition of those who may be poisoned. At the first sign of trouble, it is necessary to call the appropriate specialists.