Low birth weight babies feeding problems
Guidelines for Feeding Very Low Birth Weight Infants
1. Oxford Centre for Evidence-Based Medicine—Levels of Evidence (March 2009) [(accessed on 1 January 2015)]. Available online: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/
2. Flidel-Rimon O., Friedman S., Lev E., Juster-Reicher A., Amitay M., Shinwell E.S. Early enteral feeding and nosocomial sepsis in very low birthweight infants. Arch. Dis. Child. Fetal Neonatal Ed. 2004;89:F289–F292. doi: 10.1136/adc.2002.021923. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
3. Hartel C., Haase B., Browning-Carmo K., Gebauer C., Kattner E., Kribs A., Segerer H., Teig N., Wense A., Wieg C., et al. Does the enteral feeding advancement affect short-term outcomes in very low birth weight infants? J. Pediatr. Gastroenterol. Nutr. 2009;48:464–470. doi: 10.1097/MPG.0b013e31818c5fc3. [PubMed] [CrossRef] [Google Scholar]
4. Rochow N., Fusch G., Muhlinghaus A., Niesytto C., Straube S. , Utzig N., Fusch C. A nutritional program to improve outcome of very low birth weight infants. Clin. Nutr. 2012;31:124–131. doi: 10.1016/j.clnu.2011.07.004. [PubMed] [CrossRef] [Google Scholar]
5. McCallie K.R., Lee H.C., Mayer O., Cohen R.S., Hintz S.R., Rhine W.D. Improved outcomes with a standardized feeding protocol for very low birth weight infants. J. Perinatol. 2011;31:S61–S67. doi: 10.1038/jp.2010.185. [PubMed] [CrossRef] [Google Scholar]
6. Krishnamurthy S., Gupta P., Debnath S., Gomber S. Slow versus rapid enteral feeding advancement in preterm newborn infants 1000–1499 g: A randomized controlled trial. Acta Paediatr. 2010;99:42–46. doi: 10.1111/j.1651-2227.2009.01519.x. [PubMed] [CrossRef] [Google Scholar]
7. Dhingra A., Agrawal S.K., Kumar P., Narang A. A randomised controlled trial of two feeding schedules in neonates weighing ≤1750 g. J. Matern. Fetal Neonatal Med. 2009;22:198–203. doi: 10.1080/14767050802385749. [PubMed] [CrossRef] [Google Scholar]
8. Rudiger M., Herrmann S., Schmalisch G., Wauer R.R., Hammer H., Tschirch E. Comparison of 2-h versus 3-h enteral feeding in extremely low birth weight infants, commencing after birth. Acta Paediatr. 2008;97:764–769. doi: 10.1111/j.1651-2227.2008.00774.x. [PubMed] [CrossRef] [Google Scholar]
Rudiger M., Herrmann S., Schmalisch G., Wauer R.R., Hammer H., Tschirch E. Comparison of 2-h versus 3-h enteral feeding in extremely low birth weight infants, commencing after birth. Acta Paediatr. 2008;97:764–769. doi: 10.1111/j.1651-2227.2008.00774.x. [PubMed] [CrossRef] [Google Scholar]
9. DeMauro S.B., Abbasi S., Lorch S. The impact of feeding interval on feeding outcomes in very low birth-weight infants. J. Perinatol. 2011;31:481–486. doi: 10.1038/jp.2010.153. [PubMed] [CrossRef] [Google Scholar]
10. Morgan J., Bombell S., McGuire W. Early trophic feeding versus enteral fasting for very preterm or very low birth weight infants. Cochrane Database Syst. Rev. 2013;3:CD000504. doi: 10.1002/14651858.CD000504.pub4. [PubMed] [CrossRef] [Google Scholar]
11. Dunn L., Hulman S., Weiner J., Kliegman R. Beneficial effects of early hypocaloric enteral feeding on neonatal gastrointestinal function: Preliminary report of a randomized trial. J. Pediatr. 1988;112:622–629. doi: 10.1016/S0022-3476(88)80185-9. [PubMed] [CrossRef] [Google Scholar]
doi: 10.1016/S0022-3476(88)80185-9. [PubMed] [CrossRef] [Google Scholar]
12. Meetze W.H., Valentine C., McGuigan J.E., Conlon M., Sacks N., Neu J. Gastrointestinal priming prior to full enteral nutrition in very low birth weight infants. J. Pediatr. Gastroenterol. Nutr. 1992;15:163–170. doi: 10.1097/00005176-199208000-00011. [PubMed] [CrossRef] [Google Scholar]
13. Morgan J., Young L., McGuire W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst. Rev. 2014;12:CD001970. doi: 10.1002/14651858.CD001970.pub5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
14. Morgan J., Young L., McGuire W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst. Rev. 2014;12:CD001241. doi: 10.1002/14651858.CD001241.pub5. [PubMed] [CrossRef] [Google Scholar]
15. Schanler R.J. Outcomes of human milk-fed premature infants. Semin. Perinatol. 2011;35:29–33. doi: 10.1053/j.semperi.2010.10.005. [PubMed] [CrossRef] [Google Scholar]
Semin. Perinatol. 2011;35:29–33. doi: 10.1053/j.semperi.2010.10.005. [PubMed] [CrossRef] [Google Scholar]
16. Sullivan S., Schanler R.J., Kim J.H., Patel A.L., Trawoger R., Kiechl-Kohlendorfer U., Chan G.M., Blanco C.L., Abrams S., Cotten C.M., et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 2010;156:562–567. doi: 10.1016/j.jpeds.2009.10.040. [PubMed] [CrossRef] [Google Scholar]
17. Cristofalo E.A., Schanler R.J., Blanco C.L., Sullivan S., Trawoeger R., Kiechl-Kohlendorfer U., Dudell G., Rechtman D.J., Lee M.L., Lucas A., et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatr. 2013;163:1592–1595. doi: 10.1016/j.jpeds.2013.07.011. [PubMed] [CrossRef] [Google Scholar]
18. Schanler R.J., Lau C., Hurst N.M., Smith E.O. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics. 2005;116:400–406. doi: 10.1542/peds.2004-1974. [PubMed] [CrossRef] [Google Scholar]
Pediatrics. 2005;116:400–406. doi: 10.1542/peds.2004-1974. [PubMed] [CrossRef] [Google Scholar]
19. Ganapathy V., Hay J.W., Kim J.H. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed. Med. 2012;7:29–37. doi: 10.1089/bfm.2011.0002. [PubMed] [CrossRef] [Google Scholar]
20. Mihatsch W.A., Pohlandt F., Franz A.R., Flock F. Early feeding advancement in very low-birth-weight infants with intrauterine growth retardation and increased umbilical artery resistance. J. Pediatr. Gastroenterol. Nutr. 2002;35:144–148. doi: 10.1097/00005176-200208000-00008. [PubMed] [CrossRef] [Google Scholar]
21. Karagianni P., Briana D.D., Mitsiakos G., Elias A., Theodoridis T., Chatziioannidis E., Kyriakidou M., Nikolaidis N. Early versus delayed minimal enteral feeding and risk for necrotizing enterocolitis in preterm growth-restricted infants with abnormal antenatal Doppler results. Am. J. Perinatol. 2010;27:367–373. doi: 10.1055/s-0029-1243310. [PubMed] [CrossRef] [Google Scholar]
Perinatol. 2010;27:367–373. doi: 10.1055/s-0029-1243310. [PubMed] [CrossRef] [Google Scholar]
22. Van Elburg R.M., van den Berg A., Bunkers C.M., van Lingen R.A., Smink E.W., van E.J., Fetter W.P. Minimal enteral feeding, fetal blood flow pulsatility, and postnatal intestinal permeability in preterm infants with intrauterine growth retardation. Arch. Dis. Child. Fetal Neonatal Ed. 2004;89:F293–F296. doi: 10.1136/adc.2003.027367. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
23. Leaf A., Dorling J., Kempley S., McCormick K., Mannix P., Linsell L., Juszczak E., Brocklehurst P. Early or delayed enteral feeding for preterm growth-restricted infants: A randomized trial. Pediatrics. 2012;129:e1260–e1268. doi: 10.1542/peds.2011-2379. [PubMed] [CrossRef] [Google Scholar]
24. Kempley S., Gupta N., Linsell L., Dorling J., McCormick K., Mannix P., Juszczak E., Brocklehurst P., Leaf A. Feeding infants below 29 weeks’ gestation with abnormal antenatal Doppler: Analysis from a randomised trial. Arch. Dis. Child. Fetal Neonatal Ed. 2014;99:F6–F11. [PubMed] [Google Scholar]
Arch. Dis. Child. Fetal Neonatal Ed. 2014;99:F6–F11. [PubMed] [Google Scholar]
25. Havranek T., Madramootoo C., Carver J.D. Nasal continuous positive airway pressure affects pre- and postprandial intestinal blood flow velocity in preterm infants. J. Perinatol. 2007;27:704–708. doi: 10.1038/sj.jp.7211808. [PubMed] [CrossRef] [Google Scholar]
26. Jaile J.C., Levin T., Wung J.T., Abramson S.J., Ruzal-Shapiro C., Berdon W.E. Benign gaseous distension of the bowel in premature infants treated with nasal continuous airway pressure: A study of contributing factors. Am. J. Roentgenol. 1992;158:125–127. doi: 10.2214/ajr.158.1.1727337. [PubMed] [CrossRef] [Google Scholar]
27. Clyman R., Wickremasinghe A., Jhaveri N., Hassinger D.C., Attridge J.T., Sanocka U., Polin R., Gillam-Krakauer M., Reese J., Mammel M., et al. Enteral feeding during indomethacin and ibuprofen treatment of a patent ductus arteriosus. J. Pediatr. 2013;163:406–411. doi: 10.1016/j.jpeds.2013.01.057. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
28. Bellander M., Ley D., Polberger S., Hellstrom-Westas L. Tolerance to early human milk feeding is not compromised by indomethacin in preterm infants with persistent ductus arteriosus. Acta Paediatr. 2003;92:1074–1078. doi: 10.1111/j.1651-2227.2003.tb02580.x. [PubMed] [CrossRef] [Google Scholar]
Bellander M., Ley D., Polberger S., Hellstrom-Westas L. Tolerance to early human milk feeding is not compromised by indomethacin in preterm infants with persistent ductus arteriosus. Acta Paediatr. 2003;92:1074–1078. doi: 10.1111/j.1651-2227.2003.tb02580.x. [PubMed] [CrossRef] [Google Scholar]
29. Pezzati M., Vangi V., Biagiotti R., Bertini G., Cianciulli D., Rubaltelli F.F. Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. J. Pediatr. 1999;135:733–738. doi: 10.1016/S0022-3476(99)70093-4. [PubMed] [CrossRef] [Google Scholar]
30. Ohlsson A., Walia R., Shah S.S. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2013;4:CD003481. doi: 10.1002/14651858.CD003481.pub5. [PubMed] [CrossRef] [Google Scholar]
31. Malhotra A.K., Deorari A.K., Paul V.K., Bagga A., Singh M. Gastric residuals in preterm babies. J. Trop. Pediatr. 1992;38:262–264. doi: 10.1093/tropej/38.5.262. [PubMed] [CrossRef] [Google Scholar]
doi: 10.1093/tropej/38.5.262. [PubMed] [CrossRef] [Google Scholar]
32. Mihatsch W.A., von Schoenaich P., Fahnenstich H., Dehne N., Ebbecke H., Plath C., von Stockhausen H.B., Muche R., Franz A., Pohlandt F. The significance of gastric residuals in the early enteral feeding advancement of extremely low birth weight infants. Pediatrics. 2002;109:457–459. doi: 10.1542/peds.109.3.457. [PubMed] [CrossRef] [Google Scholar]
33. Mihatsch W.A., Franz A.R., Hogel J., Pohlandt F. Hydrolyzed protein accelerates feeding advancement in very low birth weight infants. Pediatrics. 2002;110:1199–1203. doi: 10.1542/peds.110.6.1199. [PubMed] [CrossRef] [Google Scholar]
34. Shulman R.J., Ou C.N., Smith E.O. Evaluation of potential factors predicting attainment of full gavage feedings in preterm infants. Neonatology. 2011;99:38–44. doi: 10.1159/000302020. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
35. Torrazza R.M., Parker L.A., Talaga E., Shuster J., Neu J. The value of routine evaluation of gastric residuals in very low birth weight infants. J. Perinatol. 2014 doi: 10.1038/jp.2014.147. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
J. Perinatol. 2014 doi: 10.1038/jp.2014.147. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
36. Bertino E., Giuliani F., Prandi G., Coscia A., Martano C., Fabris C. Necrotizing enterocolitis: Risk factor analysis and role of gastric residuals in very low birth weight infants. J. Pediatr. Gastroenterol. Nutr. 2009;48:437–442. doi: 10.1097/MPG.0b013e31817b6dbe. [PubMed] [CrossRef] [Google Scholar]
37. Cobb B.A., Carlo W.A., Ambalavanan N. Gastric residuals and their relationship to necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2004;113:50–53. doi: 10.1542/peds.113.1.50. [PubMed] [CrossRef] [Google Scholar]
38. Eizaguirre I., Emparanza J., Tovar J.A., Weilin W., Tapia I. Duodenogastric reflux: Values in normal children and in children with gastroesophageal reflux. Cir. Pediatr. 1993;6:114–116. (In Spanish) [PubMed] [Google Scholar]
39. Wang W., Ji S., Wang H., Wang W. 24-Hour gastroesophageal double pH monitoring acid and alkaline gastroesophageal and duodenogastric refluxes in pediatric patients.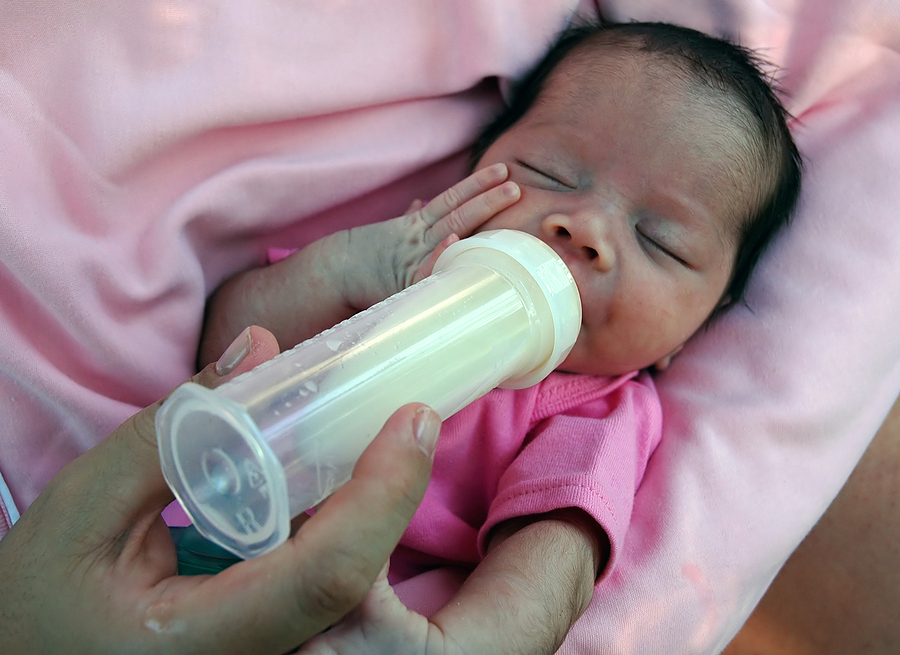 Chin. Med. J. (Engl.) 1998;111:881–884. [PubMed] [Google Scholar]
Chin. Med. J. (Engl.) 1998;111:881–884. [PubMed] [Google Scholar]
40. Bhatia P., Johnson K.J., Bell E.F. Variability of abdominal circumference of premature infants. J. Pediatr. Surg. 1990;25:543–544. doi: 10.1016/0022-3468(90)90569-U. [PubMed] [CrossRef] [Google Scholar]
41. Johnson T.S., Engstrom J.L., Gelhar D.K. Intra- and interexaminer reliability of anthropometric measurements of term infants. J. Pediatr. Gastroenterol. Nutr. 1997;24:497–505. doi: 10.1097/00005176-199705000-00001. [PubMed] [CrossRef] [Google Scholar]
42. Li Y., Lin H., Torrazza R.M., Parker L., Talaga E., Neu J. Gastric residual evaluation in preterm neonates: A useful monitoring technique or a hindrance? Pediatr. Neonatol. 2014;55:335–340. doi: 10.1016/j.pedneo.2014.02.008. [PubMed] [CrossRef] [Google Scholar]
43. De V.K., Knapp E., Al-Tawil Y., Berseth C.L. Slow infusion feedings enhance duodenal motor responses and gastric emptying in preterm infants. Am. J. Clin. Nutr. 1998;68:103–108. [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
44. Premji S., Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database Syst Rev. 2011;11:CD001819. doi: 10.1002/14651858.CD001819.pub2. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
45. Macklin D. What’s physics got to do with it? A review of the physical principles of fluid administration. J. Vasc. Access Devices. 1999;4:7–11. doi: 10.2309/108300899775970836. [CrossRef] [Google Scholar]
46. Chen S.S., Tzeng Y.L., Gau B.S., Kuo P.C., Chen J.Y. Effects of prone and supine positioning on gastric residuals in preterm infants: A time series with cross-over study. Int. J. Nurs. Stud. 2013;50:1459–1467. doi: 10.1016/j.ijnurstu.2013.02.009. [PubMed] [CrossRef] [Google Scholar]
47. Di F.J., Arko M., Herynk B., Martin R., Hibbs A.M. Characterization of cardiorespiratory events following gastroesophageal reflux in preterm infants. J. Perinatol. 2010;30:683–687. doi: 10.1038/jp.2010.27. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
2010;30:683–687. doi: 10.1038/jp.2010.27. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
48. Peter C.S., Sprodowski N., Bohnhorst B., Silny J., Poets C.F. Gastroesophageal reflux and apnea of prematurity: No temporal relationship. Pediatrics. 2002;109:8–11. doi: 10.1542/peds.109.1.8. [PubMed] [CrossRef] [Google Scholar]
49. Lopez-Alonso M., Moya M.J., Cabo J.A., Ribas J., del Carmen M.M., Silny J., Sifrim D. Twenty-four-hour esophageal impedance-pH monitoring in healthy preterm neonates: Rate and characteristics of acid, weakly acidic, and weakly alkaline gastroesophageal reflux. Pediatrics. 2006;118:e299–e308. doi: 10.1542/peds.2005-3140. [PubMed] [CrossRef] [Google Scholar]
50. Corvaglia L., Zama D., Gualdi S., Ferlini M., Aceti A., Faldella G. Gastro-oesophageal reflux increases the number of apnoeas in very preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2009;94:F188–F192. doi: 10.1136/adc.2008.143198. [PubMed] [CrossRef] [Google Scholar]
51. Corvaglia L. , Zama D., Spizzichino M., Aceti A., Mariani E., Capretti M.G., Galletti S., Faldella G. The frequency of apneas in very preterm infants is increased after non-acid gastro-esophageal reflux. Neurogastroenterol. Motil. 2011;23:303–307, e152. doi: 10.1111/j.1365-2982.2010.01650.x. [PubMed] [CrossRef] [Google Scholar]
, Zama D., Spizzichino M., Aceti A., Mariani E., Capretti M.G., Galletti S., Faldella G. The frequency of apneas in very preterm infants is increased after non-acid gastro-esophageal reflux. Neurogastroenterol. Motil. 2011;23:303–307, e152. doi: 10.1111/j.1365-2982.2010.01650.x. [PubMed] [CrossRef] [Google Scholar]
52. Snel A., Barnett C.P., Cresp T.L., Haslam R.R., Davidson G.P., Malbert T.H., Dent J., Omari T.I. Behavior and gastroesophageal reflux in the premature neonate. J. Pediatr. Gastroenterol. Nutr. 2000;30:18–21. doi: 10.1097/00005176-200001000-00012. [PubMed] [CrossRef] [Google Scholar]
53. Feranchak A.P., Orenstein S.R., Cohn J.F. Behaviors associated with onset of gastroesophageal reflux episodes in infants. Prospective study using split-screen video and pH probe. Clin. Pediatr. (Phila.) 1994;33:654–662. doi: 10.1177/000992289403301104. [PubMed] [CrossRef] [Google Scholar]
54. Barnett C.P., Omari T., Davidson G.P., Goodchild L., Lontis R., Dent J., Haslam R. R. Effect of cisapride on gastric emptying in premature infants with feed intolerance. J. Paediatr. Child Health. 2001;37:559–563. doi: 10.1046/j.1440-1754.2001.00705.x. [PubMed] [CrossRef] [Google Scholar]
R. Effect of cisapride on gastric emptying in premature infants with feed intolerance. J. Paediatr. Child Health. 2001;37:559–563. doi: 10.1046/j.1440-1754.2001.00705.x. [PubMed] [CrossRef] [Google Scholar]
55. Moore D.J., Tao B.S., Lines D.R., Hirte C., Heddle M.L., Davidson G.P. Double-blind placebo-controlled trial of omeprazole in irritable infants with gastroesophageal reflux. J. Pediatr. 2003;143:219–223. doi: 10.1067/S0022-3476(03)00207-5. [PubMed] [CrossRef] [Google Scholar]
56. Corvaglia L., Rotatori R., Ferlini M., Aceti A., Ancora G., Faldella G. The effect of body positioning on gastroesophageal reflux in premature infants: Evaluation by combined impedance and pH monitoring. J. Pediatr. 2007;151:591–596. doi: 10.1016/j.jpeds.2007.06.014. [PubMed] [CrossRef] [Google Scholar]
57. American Academy of Pediatrics Task force on sudden infant death syndrome. The changing concept of sudden infant death syndrome: Diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116:1245–1255. [PubMed] [Google Scholar]
Pediatrics. 2005;116:1245–1255. [PubMed] [Google Scholar]
58. Canadian Pediatric Society Recommendations for Safe Sleeping Environments for Infants and Children. Paediatr. Child Health. 2004;9:659–663. [PMC free article] [PubMed] [Google Scholar]
59. Cresi F., Marinaccio C., Russo M.C., Miniero R., Silvestro L. Short-term effect of domperidone on gastroesophageal reflux in newborns assessed by combined intraluminal impedance and pH monitoring. J. Perinatol. 2008;28:766–770. doi: 10.1038/jp.2008.81. [PubMed] [CrossRef] [Google Scholar]
60. Wheatley E., Kennedy K.A. Cross-over trial of treatment for bradycardia attributed to gastroesophageal reflux in preterm infants. J. Pediatr. 2009;155:516–521. doi: 10.1016/j.jpeds.2009.03.044. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
61. Djeddi D., Kongolo G., Lefaix C., Mounard J., Leke A. Effect of domperidone on QT interval in neonates. J. Pediatr. 2008;153:663–666. doi: 10.1016/j.jpeds.2008.05.013. [PubMed] [CrossRef] [Google Scholar]
62. Gunlemez A., Babaoglu A., Arisoy A.E., Turker G., Gokalp A.S. Effect of domperidone on the QTc interval in premature infants. J. Perinatol. 2010;30:50–53. doi: 10.1038/jp.2009.96. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Gunlemez A., Babaoglu A., Arisoy A.E., Turker G., Gokalp A.S. Effect of domperidone on the QTc interval in premature infants. J. Perinatol. 2010;30:50–53. doi: 10.1038/jp.2009.96. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
63. Bianconi S., Gudavalli M., Sutija V.G., Lopez A.L., Barillas-Arias L., Ron N. Ranitidine and late-onset sepsis in the neonatal intensive care unit. J. Perinat. Med. 2007;35:147–150. doi: 10.1515/JPM.2007.017. [PubMed] [CrossRef] [Google Scholar]
64. Guillet R., Stoll B.J., Cotten C.M., Gantz M., McDonald S., Poole W.K., Phelps D.L. Association of h3-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117:e137–e142. doi: 10.1542/peds.2005-1543. [PubMed] [CrossRef] [Google Scholar]
65. Terrin G., Passariello A., De C.M., Manguso F., Salvia G., Lega L., Messina F., Paludetto R., Canani R.B. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics. 2012;129:e40–e45. doi: 10.1542/peds.2011-0796. [PubMed] [CrossRef] [Google Scholar]
Pediatrics. 2012;129:e40–e45. doi: 10.1542/peds.2011-0796. [PubMed] [CrossRef] [Google Scholar]
66. Omari T.I., Haslam R.R., Lundborg P., Davidson G.P. Effect of omeprazole on acid gastroesophageal reflux and gastric acidity in preterm infants with pathological acid reflux. J. Pediatr. Gastroenterol. Nutr. 2007;44:41–44. doi: 10.1097/01.mpg.0000252190.97545.07. [PubMed] [CrossRef] [Google Scholar]
67. Davidson G., Wenzl T.G., Thomson M., Omari T., Barker P., Lundborg P., Illueca M. Efficacy and safety of once-daily esomeprazole for the treatment of gastroesophageal reflux disease in neonatal patients. J. Pediatr. 2013;163:692–698. doi: 10.1016/j.jpeds.2013.05.007. [PubMed] [CrossRef] [Google Scholar]
68. Huang R.C., Forbes D., Davies M.W. Feed thickener for newborn infants with gastro-oesophageal reflux. Cochrane Database Syst. Rev. 2002;3:CD003211. doi: 10.1002/14651858.CD003211. [PubMed] [CrossRef] [Google Scholar]
69. Gouyon J.B., Boggio V., Fantino M., Gillot I. , Schatz B., Vallin A. Smectite reduces gastroesophageal reflux in newborn infants. Dev. Pharmacol. Ther. 1989;13:46–50. [PubMed] [Google Scholar]
, Schatz B., Vallin A. Smectite reduces gastroesophageal reflux in newborn infants. Dev. Pharmacol. Ther. 1989;13:46–50. [PubMed] [Google Scholar]
70. Corvaglia L., Spizzichino M., Aceti A., Legnani E., Mariani E., Martini S., Battistini B., Faldella G. A thickened formula does not reduce apneas related to gastroesophageal reflux in preterm infants. Neonatology. 2013;103:98–102. doi: 10.1159/000342703. [PubMed] [CrossRef] [Google Scholar]
71. Woods C.W., Oliver T., Lewis K., Yang Q. Development of necrotizing enterocolitis in premature infants receiving thickened feeds using SimplyThick® J. Perinatol. 2012;32:150–152. doi: 10.1038/jp.2011.105. [PubMed] [CrossRef] [Google Scholar]
72. Clarke P., Robinson M.J. Thickening milk feeds may cause necrotising enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2004;89:F280. doi: 10.1136/adc.2003.036392. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
73. Mercier J.C., Hartmann J.F., Cohen R., Tran H., Biriotti V. , Kessler A. Intestinal occlusion and enterocolitis caused by Gelopectose. Arch. Fr. Pediatr. 1984;41:709–710. (In French) [PubMed] [Google Scholar]
, Kessler A. Intestinal occlusion and enterocolitis caused by Gelopectose. Arch. Fr. Pediatr. 1984;41:709–710. (In French) [PubMed] [Google Scholar]
74. Poets C.F., Langner M.U., Bohnhorst B. Effects of bottle feeding and two different methods of gavage feeding on oxygenation and breathing patterns in preterm infants. Acta Paediatr. 1997;86:419–423. doi: 10.1111/j.1651-2227.1997.tb09034.x. [PubMed] [CrossRef] [Google Scholar]
75. Watson J., McGuire W. Transpyloric versus gastric tube feeding for preterm infants. Cochrane Database Syst. Rev. 2013;2:CD003487. doi: 10.1002/14651858.CD003487.pub3. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
76. Malcolm W.F., Smith P.B., Mears S., Goldberg R.N., Cotten C.M. Transpyloric tube feeding in very low birthweight infants with suspected gastroesophageal reflux: Impact on apnea and bradycardia. J. Perinatol. 2009;29:372–375. doi: 10.1038/jp.2008.234. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
77. Ng E., Shah V.S. Erythromycin for the prevention and treatment of feeding intolerance in preterm infants. Cochrane Database Syst. Rev. 2008;3:CD001815. doi: 10.1002/14651858.CD001815.pub2. [PubMed] [CrossRef] [Google Scholar]
Ng E., Shah V.S. Erythromycin for the prevention and treatment of feeding intolerance in preterm infants. Cochrane Database Syst. Rev. 2008;3:CD001815. doi: 10.1002/14651858.CD001815.pub2. [PubMed] [CrossRef] [Google Scholar]
78. Mansi Y., Abdelaziz N., Ezzeldin Z., Ibrahim R. Randomized controlled trial of a high dose of oral erythromycin for the treatment of feeding intolerance in preterm infants. Neonatology. 2011;100:290–294. doi: 10.1159/000327536. [PubMed] [CrossRef] [Google Scholar]
79. Ng Y., Su P., Chen J., Quek Y., Hu J., Lee I., Lee H., Chang H. Efficacy of intermediate-dose oral erythromycin on very low birth weight infants with feeding intolerance. Pediatr. Neonatol. 2012;53:34–40. doi: 10.1016/j.pedneo.2011.11.007. [PubMed] [CrossRef] [Google Scholar]
80. Rogers S.P., Hicks P.D., Hamzo M., Veit L.E., Abrams S.A. Continuous feedings of fortified human milk deeds to nutrient losses of fat, calcium and phosphorus. Nutrients. 2010;2:230–240. doi: 10.3390/nu2030240. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
[PMC free article] [PubMed] [CrossRef] [Google Scholar]
81. Berseth C.L., Van Aerde J.E., Gross S., Stolz S.I., Harris C.L., Hansen J.W. Growth, efficacy, and safety of feeding an iron-fortified human milk fortifier. Pediatrics. 2004;114:e699–e706. doi: 10.1542/peds.2004-0911. [PubMed] [CrossRef] [Google Scholar]
82. Schanler R.J., Abrams S.A. Postnatal attainment of intrauterine macromineral accretion rates in low birth weight infants fed fortified human milk. J. Pediatr. 1995;126:441–447. doi: 10.1016/S0022-3476(95)70465-5. [PubMed] [CrossRef] [Google Scholar]
83. Schanler R.J., Shulman R.J., Lau C. Feeding strategies for premature infants: Beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics. 1999;103:1150–1157. doi: 10.1542/peds.103.6.1150. [PubMed] [CrossRef] [Google Scholar]
84. Tillman S., Brandon D.H., Silva S.G. Evaluation of human milk fortification from the time of the first feeding: Effects on infants of less than 31 weeks gestational age. J. Perinatol. 2012;32:525–531. doi: 10.1038/jp.2011.140. [PubMed] [CrossRef] [Google Scholar]
J. Perinatol. 2012;32:525–531. doi: 10.1038/jp.2011.140. [PubMed] [CrossRef] [Google Scholar]
85. Weaver L.T., Lucas A. Development of bowel habit in preterm infants. Arch. Dis. Child. 1993;68:317–320. doi: 10.1136/adc.68.3_Spec_No.317. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
86. Mihatsch W.A., Franz A.R., Lindner W., Pohlandt F. Meconium passage in extremely low birthweight infants and its relation to very early enteral nutrition. Acta Paediatr. 2001;90:409–411. doi: 10.1111/j.1651-2227.2001.tb00441.x. [PubMed] [CrossRef] [Google Scholar]
87. Shim S.Y., Kim H.S., Kim D.H., Kim E.K., Son D.W., Kim B.I., Choi J.H. Induction of early meconium evacuation promotes feeding tolerance in very low birth weight infants. Neonatology. 2007;92:67–72. doi: 10.1159/000100804. [PubMed] [CrossRef] [Google Scholar]
88. Khadr S.N., Ibhanesebhor S.E., Rennix C., Fisher H.E., Manjunatha C.M., Young D., Abara R.C. Randomized controlled trial: Impact of glycerin suppositories on time to full feeds in preterm infants. Neonatology. 2011;100:169–176. doi: 10.1159/000323964. [PubMed] [CrossRef] [Google Scholar]
Neonatology. 2011;100:169–176. doi: 10.1159/000323964. [PubMed] [CrossRef] [Google Scholar]
Low birthweight | March of Dimes
Low birthweight is when a baby is born weighing less than 5 pounds, 8 ounces.
Some low-birthweight babies are healthy, but others have serious health problems that need treatment.
Premature birth (before 37 weeks of pregnancy) and fetal growth restriction are the most common causes of low birthweight.
Being a person of color is not a cause for having a low birthweight baby. However, communities of color are disproportionately affected by racism. This affects their health and well-being and increases the risk of pregnancy complications.
Go to all your prenatal care checkups during pregnancy. Your health care provider tracks your baby’s growth and development at each visit.
Talk with your provider about what you can do to help reduce your risk for having a baby with low birthweight.
What is low birthweight?
Low birthweight is when a baby is born weighing less than 5 pounds, 8 ounces. Some babies with low birthweight are healthy, even though they’re small. But having a low weight at birth can cause serious health problems for some babies. A baby who is very small at birth may have trouble eating, gaining weight and fighting off infections. Some may have long-term health problems, too. About 1 in 12 babies (about 8 percent) in the United States is born with low birthweight.
What causes a baby to have a low birthweight?
There are two main reasons:
- Preterm birth.
- Fetal growth restriction (also called intrauterine growth restriction or small for gestational age). This means a baby doesn’t gain the weight they should before birth. Some babies may have low birthweight simply because their parents are small. Others may have low birthweight because something slowed or stopped their growth during pregnancy.
 Your health care provider measures your belly and uses ultrasound to help track your baby’s growth during pregnancy. Ultrasound uses sound waves and a computer screen to show a picture of your baby while you’re pregnant.
Your health care provider measures your belly and uses ultrasound to help track your baby’s growth during pregnancy. Ultrasound uses sound waves and a computer screen to show a picture of your baby while you’re pregnant.
If your provider thinks your baby’s growth is being restricted, you may have ultrasounds more often (every 2 to 4 weeks) to track your baby’s growth. Your provider also may do other tests such as heart rate monitoring and tests to check for infections or birth defects. Babies who have birth defects are more likely to be born too early.
Are you at risk of having a low-birthweight baby?
Some things may make you more likely than others to have a low-birthweight baby. These are called risk factors. Having a risk factor doesn’t mean you’ll definitely have a low-birthweight baby, but it may increase your chances. Talk with your health care provider about what you can do to reduce your risk.
Medical risk factors for having a low-birthweight baby
- Preterm labor.
 This is labor that starts too soon, before 37 weeks of pregnancy.
This is labor that starts too soon, before 37 weeks of pregnancy. - Chronic health conditions. These are health conditions that last for a long time or that happen again and again over a long period of time. Chronic health conditions need to be treated by a health care provider. Chronic health conditions that may lead to having a baby with low birthweight include high blood pressure, diabetes and heart, lung and kidney problems.
- Taking certain medicines to treat health conditions, such as high blood pressure, epilepsy and blood clots. Tell your provider about any prescription medicine you take. You may need to stop taking a medicine or switch to one that’s safer during pregnancy.
- Infections. Certain infections, especially infections of the internal reproductive organs during pregnancy, can slow a baby’s growth in the womb. These include cytomegalovirus, rubella, chickenpox, toxoplasmosis and certain sexually transmitted infections.

- Problems with the placenta. The placenta grows in the uterus and supplies the baby with food and oxygen through the umbilical cord. Some problems in the placenta can reduce the flow of oxygen and nutrients to the baby, which can limit the baby’s growth.
- Not gaining enough weight during pregnancy. Pregnant people who don’t gain enough weight during pregnancy are more likely to have a low-birthweight baby than those who gain the right amount of weight. If you have an eating disorder or have been treated for an eating disorder, tell your provider. Your provider can check on you and your baby carefully throughout pregnancy to help prevent complications and make sure you’re both healthy.
- Having a baby who was born too early or who had low birthweight in the past.
- Being pregnant with multiples (twins, triplets or more). More than half of multiple birth babies have low birthweight.
- Smoking, drinking alcohol, using street drugs and abusing prescription drugs. Pregnant people who smoke are more than 3 times as likely to have a baby who weighs too little at birth than people who don’t smoke. Smoking, drinking alcohol, using street drugs, and abusing prescription drugs during pregnancy can slow the baby’s growth in the womb and increase the risk for preterm birth and birth defects.
- Exposure to air pollution or lead
- Being a member of a group that experiences the effects of racism and health disparities.
- Domestic violence. This is when your partner hurts or abuses you. It includes physical, sexual and emotional abuse.
- Age. Being a teen (especially younger than 15) or being older than 35 makes you more likely than other parents to have a low-birthweight baby.
Rates of low birthweight in the United States
Black babies are more likely than others to weigh less than they should at birth. The rates of low birthweight among different ethnic groups are:
- About 1 in 7 Black babies (about 13 percent)
- About 1 in 12 Asian babies (about 8 percent)
- About 1 in 13 Native American or Alaska Native babies (about 8 percent)
- About 1 in 14 Latinx babies (about 7 percent)
- About 1 in 14 White babies (about 7 percent)
March of Dimes recognizes that racism and its effects are factors in the health disparities in pregnancy outcomes and babies’ health. We must work together to bring fair, just and full access to health care for all moms and babies.
Does a low birth weight cause problems for the baby?
Yes. Babies who weigh less than they should at birth are more likely than babies whose weight is normal to have health problems. Some need special care in a hospital’s newborn intensive care unit (also called NICU) to treat medical problems. These include:
These include:
- Breathing problems, such as respiratory distress syndrome (also called RDS). Babies with RDS don’t have a protein called surfactant that keeps small air sacs in a baby’s lungs from collapsing. Treatment with surfactant helps these babies breathe more easily. Babies who have RDS also may need oxygen and other breathing help to make their lungs work.
- Bleeding in the brain (also called intraventricular hemorrhage or IVH). Most brain bleeds are mild and go away on their own. More severe bleeds can cause pressure on the brain that can cause fluid to build up in the brain. This can cause brain damage. In some cases, a surgeon may insert a tube into the baby’s brain to drain the fluid.
- Patent ductus arteriosus. Patent ductus arteriosus is when an opening between 2 major blood vessels leading from the heart does not close properly. This can cause extra blood to flow to the lungs. In many babies who have patent ductus arteriosus, the opening closes on its own within a few days after birth.
 Some babies need medicine or surgery to close the opening.
Some babies need medicine or surgery to close the opening. - Necrotizing enterocolitis. This is a problem in a baby’s intestines. The intestines are long tubes that are part of the digestive system. The digestive system helps the body break down food. Necrotizing enterocolitis can be dangerous for a baby and can cause feeding problems, swelling in the belly, and other complications. Babies who have necrotizing enterocolitis are treated with antibiotics and fed through an intravenous, or IV, tube. Some babies need surgery to remove damaged parts of intestine.
- Retinopathy of prematurity. This eye disease is what happens when a baby’s retinas don’t fully develop in the weeks after birth.
- Jaundice. This is a condition that makes a baby’s eyes and skin look yellow. It’s caused when there’s too much of a substance called bilirubin in the blood.
- Infections. The immune system protects the body from infection.
 In a baby who is born too early, the immune system may not be fully developed and may not be able to fight off infection.
In a baby who is born too early, the immune system may not be fully developed and may not be able to fight off infection.
Does a low weight at birth cause problems later in life?
Babies who are born weighing too little may be more likely than others to have certain health conditions later in life, including:
- Diabetes
- Heart disease
- High blood pressure
- Intellectual and developmental disabilities
- Metabolic syndrome
- Obesity
If you’ve had a baby who weighed less than they should have at birth, talk with their health care provider about what you can do to help your baby be healthy. As your child grows, make sure they eat healthy food, stay active and go to all their health care checkups. Regular checkups can help your baby’s provider spot health conditions that may cause problems as your baby grows older. These checkups also help make sure that your child gets all the vaccinations they need to stay protected from certain harmful diseases.
If my baby has developmental delays, do they need early intervention services?
Yes. If your baby has developmental delays, it’s important to get early intervention services as soon as possible. Developmental delays are when your child doesn’t reach developmental milestones when expected. Early intervention services can help improve your child’s development. They can help children from birth through 3 years old learn important skills. Services include therapy to help a child talk, walk, learn self-help skills and interact with others.
The CDC program Learn the signs. Act early offers tools and information for parents who think their child may have developmental delays. You can find your state’s contact information for early intervention services. You don’t need a doctor’s referral or a medical diagnosis to ask for a free screening.
Last reviewed: June, 2021
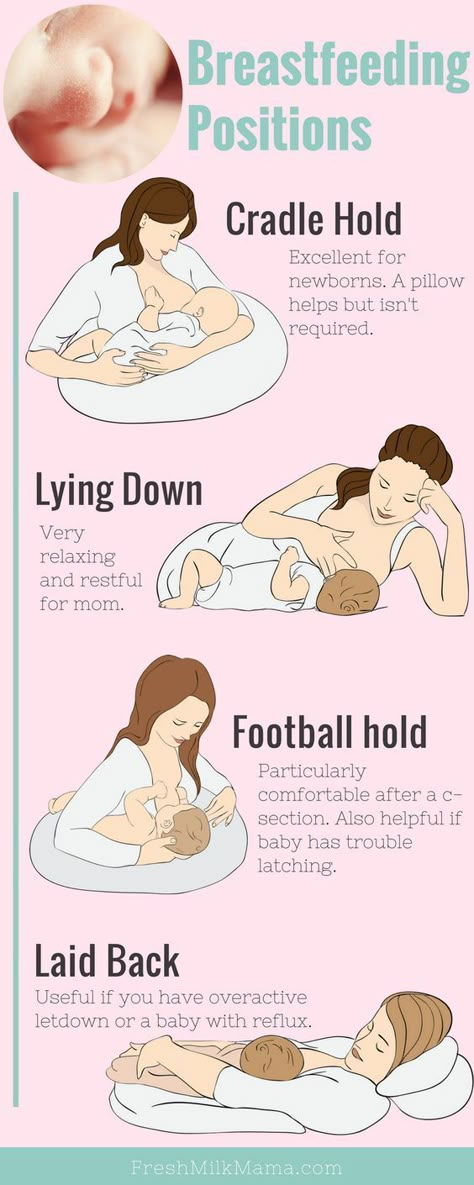
Feeding premature babies | Breastfeeding premature babies
Premature babies have a special need for breast milk, but it can be difficult to breastfeed them directly. Our expert advice will help you provide your premature baby with healthy breast milk.
Our expert advice will help you provide your premature baby with healthy breast milk.
Share this information
Professor Katsumi Mizuno, Department of Pediatrics, Showa University Koto Toyosu Hospital:
Katsumi is a Certified Breastfeeding Consultant, Professor of Pediatrics at Showa Medical University, and one of Japan's leading pediatric neonatologists. His research focuses on neonatal suckling skills, breast milk banking, and the use of breast milk for feeding premature babies in neonatal intensive care units.
Babies born before the 37th week of pregnancy are considered premature. 1 The causes of preterm birth are not always obvious, but certain factors increase the likelihood of such an event. These include: twin or multiple pregnancy, certain diseases of the mother or fetus, as well as a history of premature birth. nine0003
Because premature babies spend less time in the womb, they are not mature enough and may be more susceptible to infection and disease. They often require hospitalization in the neonatal intensive care unit.
They often require hospitalization in the neonatal intensive care unit.
Why is breast milk so important for premature babies?
Breast milk is essential for optimal growth and development of term babies, but it is even more important for premature babies.
During pregnancy, the fetus receives important substances from the mother through the placenta, such as DHA (a fatty acid essential for brain and eye development) and immunoglobulin G (an antibody). nine0012 2.3 A premature infant did not receive all of these substances. However, the milk produced by a premature mother contains more fat and secretory immunoglobulin than mothers of full-term babies. 4
In addition, premature babies have an underdeveloped gastrointestinal tract, which can make digestion and absorption of nutrients difficult, so they need food that their sensitive stomach and intestines can easily digest. Breast milk contains enzymes that make it easier for the baby to digest, 5 as well as epidermal growth factor, which accelerates the development of the gastrointestinal tract 6 . Premature infants who are predominantly breastfed have much lower intestinal permeability than formula-fed infants, meaning fewer potentially harmful particles from the stomach and intestines enter their bloodstream. 7
Premature infants who are predominantly breastfed have much lower intestinal permeability than formula-fed infants, meaning fewer potentially harmful particles from the stomach and intestines enter their bloodstream. 7
Breast milk is so important for premature babies that if the baby's mother does not produce enough breast milk at first for any reason, it is recommended that the deficiency be replenished with donor milk rather than formula. nine0003
Does breast milk improve the condition of premature babies?
Breast milk contains protective substances that can prevent serious diseases that preterm infants are susceptible to, 8 such as severe infections, 9 retinopathy of prematurity (which can cause vision loss) 10 and bronchopulmonary dysplasia (chronic lung disease). 11
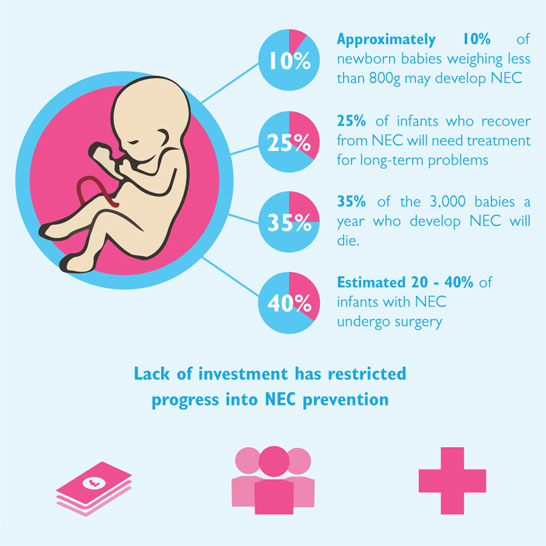
The more milk your baby gets, the lower the risk of developing diseases. nine0012 12 Every additional 10 ml of milk per kilogram of body weight per day reduces the risk of sepsis by 19%.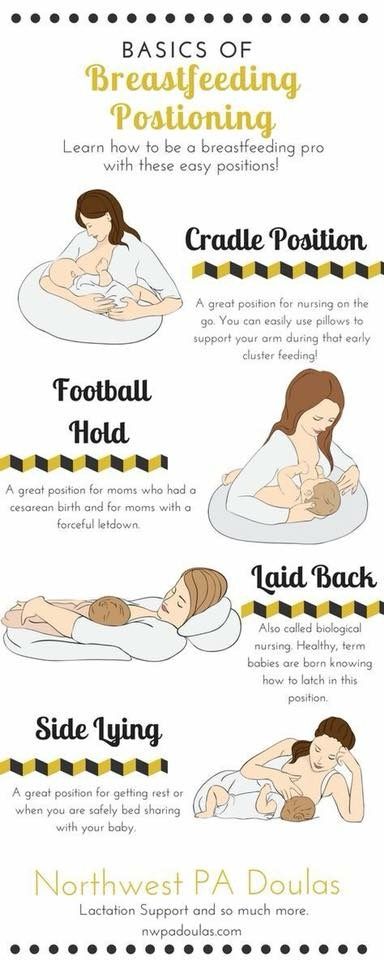 9 The risk of developing necrotizing enterocolitis (a potentially fatal bowel disease) in premature infants who are breastfed is ten times lower than those who are formula fed. 13 That's why every drop counts!
9 The risk of developing necrotizing enterocolitis (a potentially fatal bowel disease) in premature infants who are breastfed is ten times lower than those who are formula fed. 13 That's why every drop counts!
Most importantly, premature infants who are breastfed are typically discharged an average of two weeks earlier than formula-fed infants. nine0012 14 They also have a 6% lower risk of readmission in the first year of life. 15
Breast milk has been proven to have a beneficial effect on mental and physical development in the long term. Studies show that low-birth-weight babies who are breastfed in the neonatal intensive care unit have an average IQ of up to five points higher than those who are not breastfed. 15 In addition, their cardiovascular system works better during their lifetime. nine0012 17
Will milk be produced if the baby is born prematurely?
Yes, the mother's body is ready to produce milk by the middle of pregnancy. After the baby is born and the placenta is born, the level of progesterone, the pregnancy hormone, drops, and the production of colostrum, the first milk, starts in the breast. This usually happens after the newborn is put to the breast and begins to suckle rhythmically, but if the baby was born prematurely, he most likely will not be able to latch on at first. nine0003
After the baby is born and the placenta is born, the level of progesterone, the pregnancy hormone, drops, and the production of colostrum, the first milk, starts in the breast. This usually happens after the newborn is put to the breast and begins to suckle rhythmically, but if the baby was born prematurely, he most likely will not be able to latch on at first. nine0003
To replicate the sensations that trigger milk production, you can manually stimulate the breasts and nipples, or use a breast pump to express nutrient-rich colostrum for your baby. 18 Read below for more information on what to do if your premature baby is not yet able to breastfeed.
Breast milk usually comes in two to four days after birth, but if it was premature, the milk supply may be delayed. However, a recent study shows that moms who started pumping within one hour of giving birth had milk coming in as expected. nine0012 19 This is why it is important to start expressing breast milk as early as possible.
How to prepare if the baby is expected prematurely?
Visit the neonatal intensive care unit to see how it works and how premature babies are cared for. In addition, it will be useful to learn how breast milk is produced and secreted and why it is not only a healthy food, but also an important medicine for premature babies. Read more about this in our free e-book Surprising Breast Milk Facts .
What if a premature baby cannot breastfeed?
Many babies born before 34 weeks have difficulty coordinating sucking, swallowing and breathing. Until the baby masters these skills, nurses will feed him through a tube that is inserted into the nose or mouth and provides food directly into the stomach. In this way, the baby can be fed continuously until he is ready to breastfeed.
If your baby is too weak to latch on and suckle milk, you can use a breast pump* available at the hospital or maternity hospital to “do the job for the baby”. Breast stimulation with research-based technology, 20 mimics the rhythm of the baby's suckling, plays an important role in starting and maintaining milk production in the first hours after birth 21 .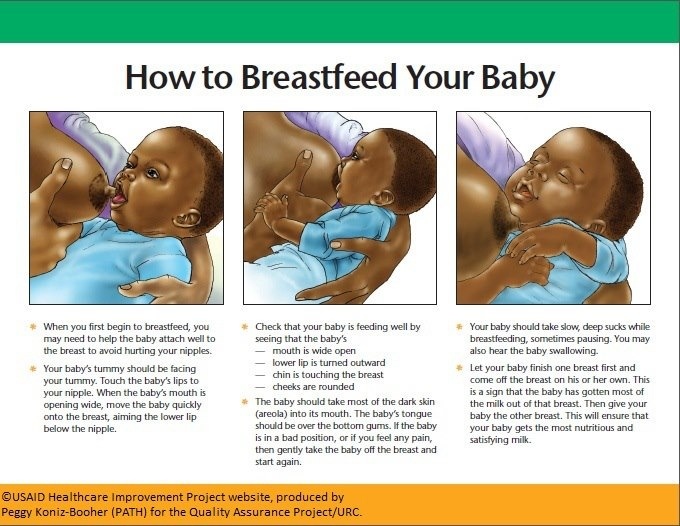
Milk should be expressed at the same frequency as term infants are usually fed every two to three hours, i.e. 8 to 12 times a day.
You can try putting a small amount of expressed breast milk into the baby's mouth with a syringe, or putting milk-soaked cotton swabs in the baby's mouth. 22 This is how your baby learns the taste of your milk, which will facilitate the transition to breastfeeding in the future. In addition, the protective substances that make up breast milk will help strengthen the local immunity of the baby's oral cavity. You can be involved in the care of your premature baby in a variety of ways - check with your healthcare provider for details.
Very low birth weight babies - less than 1.5 kg - usually need extra protein, calcium and phosphorus, so they are given fortified breast milk. In some countries, such additives are made on human milk, and, for example, in Japan, on cow's milk. nine0003
Recommendations for pumping milk
If the baby will be in the neonatal intensive care unit for a long time, neonatologists recommend using a double breast pump for pumping. I always recommend Medela Symphony*. Double pumping not only speeds up the process, but also produces an average of 18% more milk than pumping from each breast in turn. 23
I always recommend Medela Symphony*. Double pumping not only speeds up the process, but also produces an average of 18% more milk than pumping from each breast in turn. 23
In addition, I advise you to create the most comfortable conditions for pumping. It is generally agreed that it is best to express milk during or after prolonged skin-to-skin contact with the baby (more on this "kangaroo method" below). Another good option is to sit next to the crib and watch your baby while he pumps. Oxytocin (the hormone that stimulates milk flow) is released when you look at your baby, touch him, smell him and think about him, 24 Therefore, comfortable and calm conditions must be created for this in the neonatal intensive care unit.
What is kangaroo care for premature babies?
The so-called kangaroo method involves prolonged skin-to-skin contact between parents and infant. This is extremely beneficial for you and your baby, as well as for milk production. Skin-to-skin contact normalizes the baby's breathing and heartbeat, keeps him warm and allows him to be as close to the parent as possible. Kangaroo care is believed to have a beneficial effect on the health of premature babies, 25 and it helps mothers express more milk 26 and breastfeed longer. 27 Skin-to-skin contact 30-60 minutes before feeding gives baby time to wake up and be hungry so he can eat without being forced.
Skin-to-skin contact normalizes the baby's breathing and heartbeat, keeps him warm and allows him to be as close to the parent as possible. Kangaroo care is believed to have a beneficial effect on the health of premature babies, 25 and it helps mothers express more milk 26 and breastfeed longer. 27 Skin-to-skin contact 30-60 minutes before feeding gives baby time to wake up and be hungry so he can eat without being forced.
What if the neonatal intensive care unit offers formula feeding?
Feel free to state that you want to breastfeed your baby instead of formula. If you don't have enough breast milk to feed your baby, ask the ward for help to increase your milk supply. nine0003
It is natural for mothers whose babies are in the neonatal intensive care unit to experience anxiety and stress. Sometimes these experiences interfere with milk production, so it's important to ask for any help you may need. Remember that you have the right to seek support.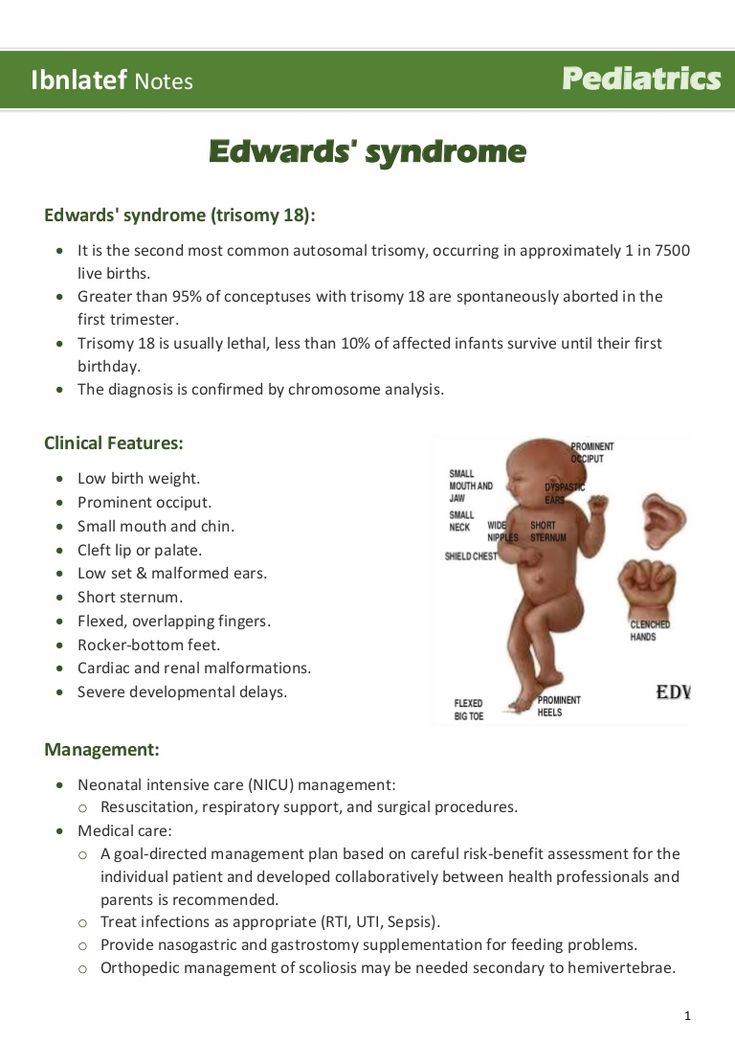 Your healthcare provider may be able to recommend a suitable lactation specialist, such as a lactation consultant, for you.
Your healthcare provider may be able to recommend a suitable lactation specialist, such as a lactation consultant, for you.
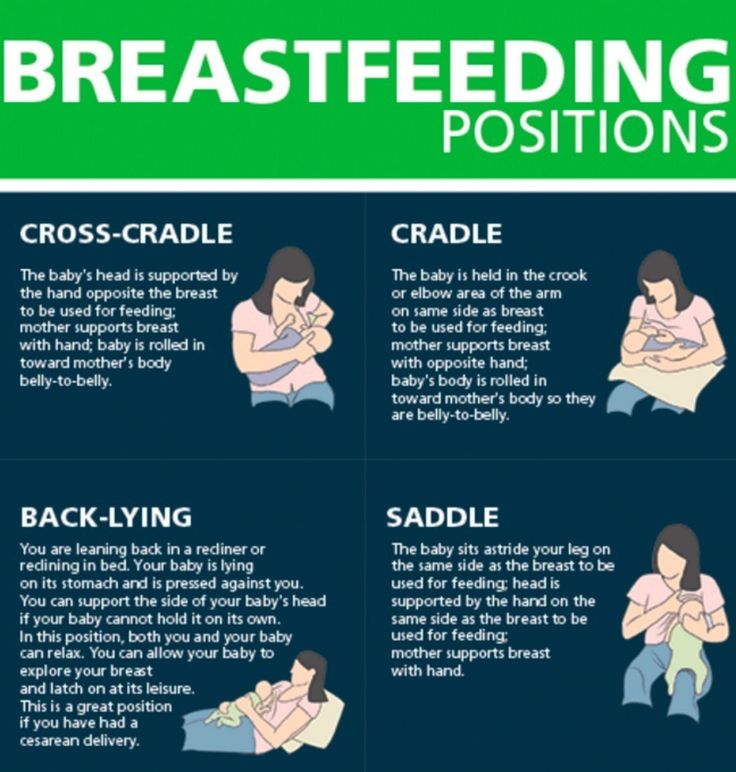
How to switch from pumping to breastfeeding? nine0018
At whatever gestational age a baby is born, if the baby is stable enough for skin-to-skin contact, it can seek the breast for sedative suckling. This is the perfect way for your baby to learn sucking skills before they learn to coordinate sucking, swallowing and breathing.
Babies love the smell of breastmilk, so you can put some milk on the nipple before putting your baby to the breast to make him want to suckle. He might even be able to suck some milk. Don't worry if your baby seems to suck very little - he learns every time. He can start with one or two sips and gradually move on to full breastfeeding. Until then, the baby can be fed through a tube, pressed to the breast, so that the taste of milk and touching the breast is associated with a feeling of satiety. nine0003
You can put your baby to the breast for sedative suckling as soon as you are ready for kangaroo care, unless your baby is suffering from bradycardia (slow heartbeat) or low oxygen levels in the blood. You can switch to breastfeeding as soon as the baby is ready for it. Gradually, he will gain enough strength to suckle longer and suck out more milk.
You can switch to breastfeeding as soon as the baby is ready for it. Gradually, he will gain enough strength to suckle longer and suck out more milk.
Literature
1 World Health Organization. Geneva, Switzerland; 2018. Media Centre: Preterm birth fact sheet; November 2017 [03/26/2018]. Available from : http://www.who.int/mediacentre/factsheets/fs363/en/ - World Health Organization. Geneva, Switzerland; 2018. "Media Center: Prematurity Fact Sheet"; November 2017 [3/26/2018]. Article at: http://www.who.int/mediacentre/factsheets/fs363/en/
2 Duttaroy AK. Transport of fatty acids across the human placenta: a review. nine0085 Prog Lipid Res . 2009;48(1):52-61. - Duttaroy A.K., "Transfer of fatty acids across the human placenta: a review". Prog Lipid Res. 2009;48(1):52-61.
3 Palmeira P et al. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012: 985646. - Palmeira P. et al., Placental transfer of immunoglobulin G through the placenta with healthy and pathological pregnancy. " Klin Virgo Immunol. 2012: 985646.
IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012: 985646. - Palmeira P. et al., Placental transfer of immunoglobulin G through the placenta with healthy and pathological pregnancy. " Klin Virgo Immunol. 2012: 985646.
4 Underwood Ma. Human Milk Formature North Am . 2013;60(1):189-207. - Underwood, M.A., "Breast milk for the premature baby." 1):189-207.
5 Pamblanco M et al. Bile salt - stimulated lipase activity in human colostrum from mothers of infants of different gestational age and birthweight. Acta Paediatr. 1987;76(2):328-331. - Pamblanco M. et al., "Bile salt-activated lipase and its activity in colostrum of mothers of infants of various gestational ages and birth weights. " Akta Pediatr. 1987;76(2):328-331.
" Akta Pediatr. 1987;76(2):328-331.
6 Dvorak B. Milk epidermal growth factor and gut protection. J Pediatr. 2010;156(2): S 31-35. - Dvorak B., "Epidermal growth factor in milk and gut protection". F Pediatrician (Journal of Pediatrics). 2010;156(2):S31-35.
7 Taylor SN et al. Intestinal permeability in preterm infants by feeding type: mother's milk versus formula. Breastfeed Med . 2009;4(1):11-15.- Theilon S.N. et al., "Intestinal permeability in preterm infants and its association with type of feeding: breast milk or formula." Brestfeed Med (Breastfeeding Medicine). 2009;4(1):11-15.
8 Newburg DS. Innate immunity and human milk. J Nutr . 2005;135(5):1308-1312. — Newburgh, D.S., "Natural Immunity and Breast Milk." F Int. 2005;135(5):1308-1312. nine0085
nine0085
9 Patel AL et al. Impact of early human milk on sepsis and health-care costs in very low birth weight infants. J Perinatol . 2013;33(7):514-519.- Patel A.L. et al., "Impact of early breast milk on sepsis and health care costs in extremely low birth weight infants". Zh Perinatol (Journal of Perinatology). 2013;33(7):514-519.
10 Zhou J et al . Human milk feeding as a protective factor for retinopathy of prematurity: a meta-analysis. Pediatrics. 2015;136(6): e 1576-1586. - Zhou Q. et al., "Breastfeeding as a protective factor against retinopathy of prematurity: a meta-analysis." Pediatrix (Pediatrics). 2015;136(6):e1576-1586.
11 Patel AL et al. Influence of own mother's milk on bronchopulmonary dysplasia and costs. 12 MEIER PP ET AL AL AL AL . Improving the use of human milk during and after the NICU stay. Clin Perinatol. 2010;37(1):217-245. - Meyer P.P. et al., "Optimizing the use of breast milk during and after a stay in the neonatal intensive care unit." nine0085 Perinatol wedge. (Clinical perinatology). 2010;37(1):217-245. 13 Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 14 Schanler RJ et al. Randomized trial of donor human milk versus preterm formula as substitutes for mothers' own milk in the feeding of extremely premature infants. nine0085 Pediatrics. 2005;116(2):400-406. - Chanler R.J. et al., "Randomized Trial of Donor Human Milk Versus Prematurity Formula as a Breast Milk Substitute in Severely Preterm Infants". Pediatrix (Pediatrics). 2005;116(2):400-406. 15 Vohr BR et al. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. nine0085 Pediatrics. 2006;118(1): e 115-123. - Thief B.R. et al., Developmental Beneficial Effects of Breast Milk in the Intensive Care Unit on Extremely Low Birth Weight Infants by 18 Months of Age. 16 Victora CG et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. nine0085 2016;387(10017):475-490. - Victor S.J. et al., "Breastfeeding in the 21st century: epidemiology, mechanisms and long-term effects". Lancet (Lancet). 2016;387(10017):475-490. 17 Lewandowski AJ et al. Breast milk consumption in preterm neonates and cardiac shape in adulthood. Pediatrics. 2016;138(1): pii : e 20160050. - Lewandowski, A.J. et al., "Breastfeeding in preterm infants and cardiovascular health in adulthood." nine0085 Pediatrix (Pediatrics). 2016;138(1):pii:e20160050. 18 Meier PP et al. Which breast pump for which mother: an evidence-based approach to individualizing breast pump technology. J. Perinatol. 2016;36(7):493-499. 19 Parker LA et al. Effect of early breast milk expression on milk volume and timing of lactogenesis stage II among mothers of very low birth weight infants: a pilot study. J Perinatol. 2012;32(3):205-209. - Parker L.A. et al., "Effect of early pumping on milk supply and timing of the second stage of lactogenesis in mothers of extremely low birth weight infants: a pilot study." J Perinatol (Journal of Perinatology). 2012;32(3):205-209. 20 Meier PP et al. Breast pump suction patterns that mimic the human infant during breastfeeding: greater milk output in less time spent pumping for breast pump-dependent mothers with premature infants. J Perinatol. 2012;32(2):103-110. - Meyer P.P. et al., "Pumping patterns that mimic breastfeeding behavior: more milk and less time for constantly pumping mothers of preterm infants. 21 Parker LA et al. Association of timing of initiation of breastmilk expression on milk volume and timing of lactogenesis stage II among mothers of very low-birth-weight infants. Breastfeed Med . 2015;10(2):84-91. - Parker L.A. et al., "Effect of early pumping on milk supply and timing of the second stage of lactogenesis in mothers of extremely low birth weight infants: a pilot study." nine0085 Brestfeed Med (Breastfeeding Medicine). 2015;10(2):84-91. 22 Lee J et al. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics. 2015;135(2): e 357-366. - Lee J. et al., "Oropharyngeal colostrum ingestion in very preterm infants: a randomized controlled clinical trial." Pediatrix (Pediatrics). 2015;135(2):e357-366. nine0085 23 Prime PK et al. 24 Uvn ä s Moberg K Oxytocin effects in mothers and infants during breastfeeding. Infant 2013; 9(6):201–206. - Uvenas-Moberg K, Prime DK, "Oxytocin effects on mother and child during breastfeeding". Infant. 2013;9(6):201-206. 25 Boundy EO et al. nine0085 Kangaroo mother care and neonatal outcomes: a meta-analysis. Pediatrics. 2015;137(1): e 20152238. - Boundi I.O. and co-authors, "The Kangaroo Method and Its Impact on Newborns: A Meta-Analysis". 26 Acuña-Muga J et al. Volume of milk obtained in relation to location and circumstances of expression in mothers of very low birth weight infants. nine0085 J Hum Lact . 2014;30(1):41-46 - Akunya-Muga, J. et al., "The amount of milk expressed by location and circumstances of pumping in mothers of extremely low birth weight infants." F Hum Lakt. 2014;30(1):41-46 27 Nyqvist KH et al. Towards universal kangaroo mother care: recommendations and report from the first European conference and seventh international workshop on kangaroo mother care. nine0085 Acta Paediatr . 2010;99(6):820-826.- Nukvist K.H. et al., "On the Universality of the Kangaroo Method: Recommendations and Report from the First European Conference and the Seventh International Kangaroo Method Workshop". 28 American Academy of Pediatrics - Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics . 2012;129(3): e 827-841.- American Academy of Pediatrics - Section "Breastfeeding", "Breastfeeding and the use of breast milk". Pediatrix (Pediatrics). 2012;129(3): e 827-841. *RC No. FZZ 2010/06525 dated 03/17/2021 The birth of a small child is not uncommon today. Often, such babies are born on time or a little earlier, but due to a lack of weight, they can significantly lag behind their peers in development. Pediatricians and neuropathologists closely monitor the child's condition, because a child's body weight deficiency is a risk factor for changes in the neurological status, functional disorders of the cardiovascular and autonomic nervous systems. To assess the development of your child and the compliance with the norm of the main indicators (height, weight), you can contact a pediatrician or independently - according to existing tables. In the first months, the child is actively growing, adding up to 25-60 grams per day. Small children with adequate nutrition can increase body weight more intensively than their peers. For the first month of life, children should gain up to 1.3-1.7 kg. After 5-6 months of life, the intensity of weight gain decreases somewhat - in 30 days, the increase can be only 400-700 grams. nine0003 The length of the child's body during the first month increases by 4-7 cm, and after 5-6 months of life, growth is added less intensively - by 2-3 cm. The main cause of underweight in the neonatal period is the refusal of the baby to suckle. Small children have poor appetite and spend most of the day sleeping. Often, parents have to wake up the child for a long time, and after a few minutes of sucking on the breast or a bottle of formula, the newborn falls asleep again. Children are especially sleepy, in whom pronounced physiological jaundice was observed in the first days of life. nine0003 As a result, after the next weighing, the doctor can tell the mother that the newborn has not gained weight at all or the increase is insignificant. If the situation does not improve for several months, the mother and baby may be hospitalized for a comprehensive examination and tube feeding in a hospital setting. Sometimes the cause of low weight gain lies in non-compliance with breastfeeding tactics. Pediatricians recommend applying the baby to only one breast during feeding so that it sucks out the "hind" milk, which is of particular energy value and rich in nutrients. Due to their inexperience, mothers offer both breasts to newborns. In this case, the child sucks the upper milk without making any effort and quickly falls asleep, slightly satisfying his hunger. nine0003 If the baby has had an infectious disease, has been ill for a long time, suffered from a high temperature or an intestinal disorder, then the monthly weight gain may be significantly less than usual. In this case, the timing of the introduction of complementary foods is also shifted, and during the period of illness, in general, many children practically refuse to eat, which is reflected in their weight. Parents should actively communicate with the pediatrician, if necessary, ask him questions of interest and adhere to all recommendations. If you are breastfeeding, pay special attention to your diet. Drink as much liquid as possible: low-fat milk, compotes, hypoallergenic juices. Your diet must include boiled or baked meat. Take extra vitamins (as advised by the doctor). Breastfeed your baby immediately after waking up, when he is active, in a good mood and does not want to sleep. nine0003 But sometimes women's milk is produced in insufficient quantities or the baby does not have enough strength to suck it out. In this case, it is necessary to start supplementing with special infant formula as soon as possible. For children prone to allergic reactions, special hypoallergenic products are intended, which can be bought at a pharmacy, having previously discussed the mixture option with a pediatric nutritionist or pediatrician. Small babies are not adapted to intensive sucking, so the nipple on the bottle must be soft and pliable so that the child can fill up without problems. In addition, in order to increase the rate of weight gain and, accordingly, for the proper growth and development of the child, it is recommended to give courses of preparations containing L-carnitine (levorcarnitine), an essential vitamin-like substance that has anabolic properties and has proven itself to normalize body weight in case of its deficiency. In addition, by increasing the secretory and enzymatic activity of gastric and intestinal juices, appetite and digestion improve. One of these drugs is Elkar, containing an aqueous solution of L-carnitine. Elkar is included in the "National program for optimizing the feeding of children in the first year of life" as a means of correcting malnutrition of the II degree. nine0003 In children, in contrast to the adult body, where levocarnitine is among the substances produced, the synthesis of this compound covers only 1% of the required amount. Of course, the required amount of L-carnitine is found in breast milk, but if natural feeding is impaired or impossible, the drug must be added to the diet. In underweight children, psychomotor development is often retarded, which can subsequently manifest itself in the form of speech defects, instability of the nervous system. Elcar improves the energy supply of brain activity, which will help to avoid or reduce the degree of development of functional failure in various areas of the child's neuropsychic response (motor, emotional-motivational, vegetative, cognitive spheres). nine0003 Another very important point: levocarnitine improves immunity, which is vital for small children, since almost all of them are predisposed to the development of infectious diseases. The rate of weight gain is influenced by many external and internal factors. The task of parents is to help the crumbs get stronger as soon as possible. Walk more with your child in the fresh air so that his body receives the necessary amount of oxygen. And don't forget to visit your pediatrician. Small children need professional medical supervision and the attention of loved ones. Arch DIS Child Neonat ED . 2017;102(3): F 256- F 261. - Patel A.L. et al., "Effect of breast milk on bronchopulmonary dysplasia and health care costs." Arch Dis Child Fetal Neonate Ed. 2017;102(3): F 256- F 261.
Arch DIS Child Neonat ED . 2017;102(3): F 256- F 261. - Patel A.L. et al., "Effect of breast milk on bronchopulmonary dysplasia and health care costs." Arch Dis Child Fetal Neonate Ed. 2017;102(3): F 256- F 261.  1990;336(8730-8731):1519-1523. — Lucas A, Cole TJ, "Breast milk and neonatal necrotizing enterocolitis." Lancet 1990;336(8730-8731):1519-1523.
1990;336(8730-8731):1519-1523. — Lucas A, Cole TJ, "Breast milk and neonatal necrotizing enterocolitis." Lancet 1990;336(8730-8731):1519-1523.  Pediatrix (Pediatrics). 2006;118(1):e115-123.
Pediatrix (Pediatrics). 2006;118(1):e115-123. 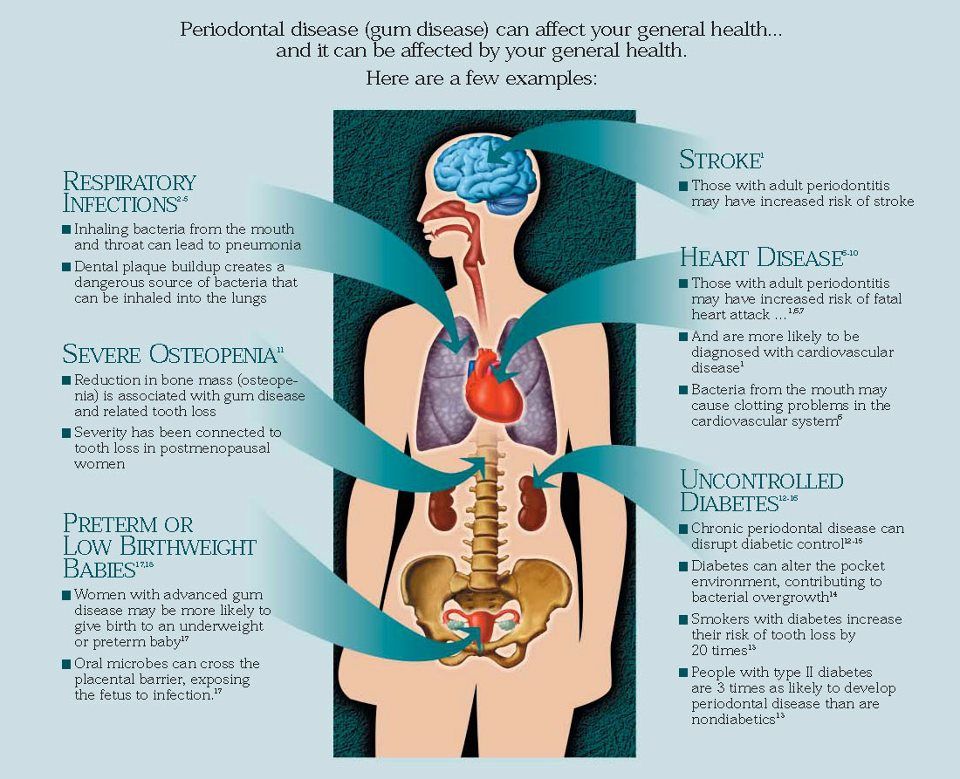 - Meyer P.P. et al., Breastpump Selection: A Scientific Approach to Customizing Pumping Technology. J Perinatol (Journal of Perinatology). 2016;36(7):493-499.
- Meyer P.P. et al., Breastpump Selection: A Scientific Approach to Customizing Pumping Technology. J Perinatol (Journal of Perinatology). 2016;36(7):493-499.  " nine0085 J Perinatol (Journal of Perinatology). 2012;32(2):103-110.
" nine0085 J Perinatol (Journal of Perinatology). 2012;32(2):103-110.  Simultaneous breast expression in breastfeeding women is more efficacious than sequential breast expression. Breastfeed Med 2012; 7(6):442–447. - Prime D.K. and co-authors. "During the period of breastfeeding, simultaneous pumping of both breasts is more productive than sequential pumping." Brestfeed Med (Breastfeeding Medicine). 2012;7(6):442-447.
Simultaneous breast expression in breastfeeding women is more efficacious than sequential breast expression. Breastfeed Med 2012; 7(6):442–447. - Prime D.K. and co-authors. "During the period of breastfeeding, simultaneous pumping of both breasts is more productive than sequential pumping." Brestfeed Med (Breastfeeding Medicine). 2012;7(6):442-447.  Pediatrix (Pediatrics). 2015;137(1): e20152238.
Pediatrix (Pediatrics). 2015;137(1): e20152238.  Akta Pediatr. 2010;99(6):820-826.
Akta Pediatr. 2010;99(6):820-826. Read instructions before use. Consult a specialist about possible contraindications.
how to help them develop?
 But because of their weakness, underweight children do not eat well, and the rate of weight gain in children born with low body weight determines their further physical and psychomotor development and the formation of the immune system. nine0003
But because of their weakness, underweight children do not eat well, and the rate of weight gain in children born with low body weight determines their further physical and psychomotor development and the formation of the immune system. nine0003 How much should a newborn gain in weight?
 But parents should understand that these figures are approximate. Each child is individual. Its weight and height depend on many factors: heredity, the quality of the mother's nutrition, the state of health of the newborn, the severity of childbirth.
But parents should understand that these figures are approximate. Each child is individual. Its weight and height depend on many factors: heredity, the quality of the mother's nutrition, the state of health of the newborn, the severity of childbirth. Why is the child not gaining weight well?

How to help a child gain weight and catch up with their peers in their development?
 nine0003
nine0003