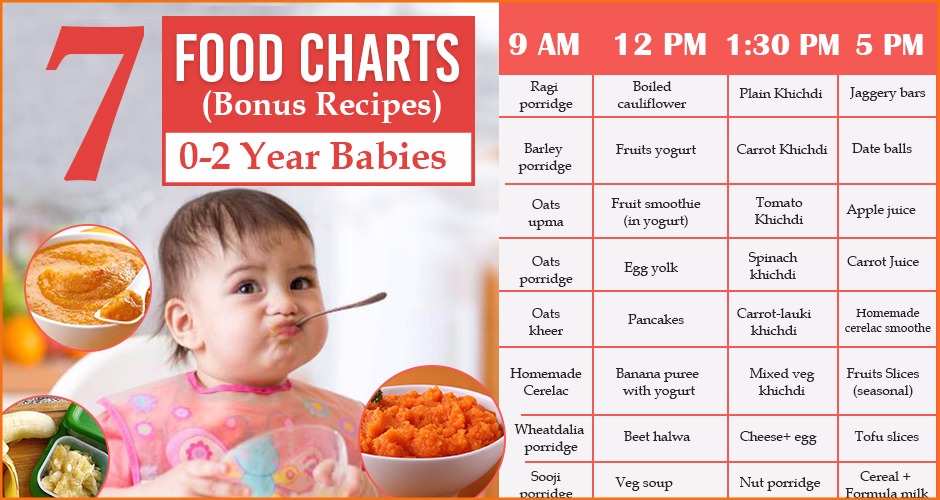
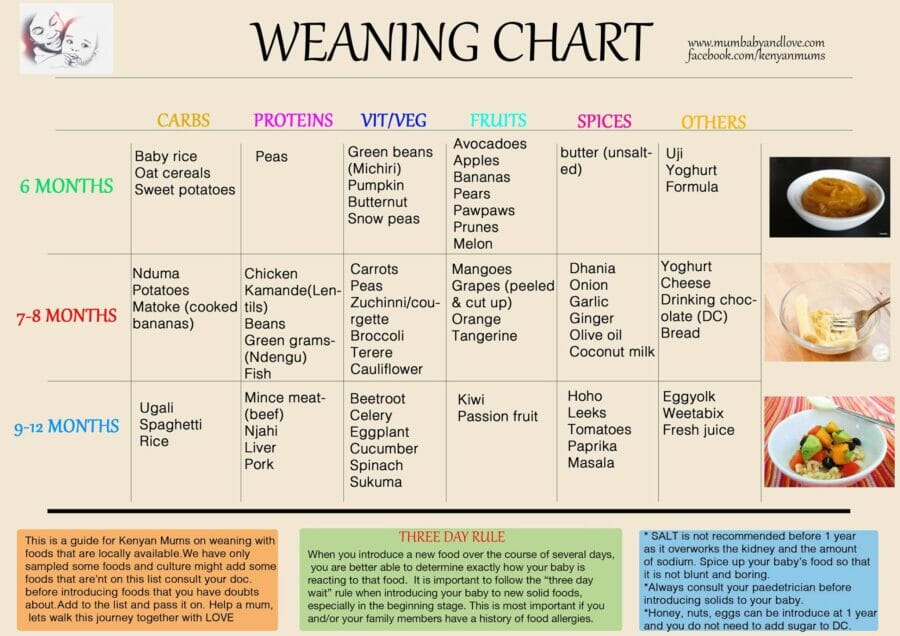
Usa today baby food
Here's how to make homemade baby food for your bundle of joy
If your baby is about to celebrate their half-birthday, it’s time to think about introducing solid foods.
While many continue to breastfeed or formula feed for the first year or two, when an infant has reached six months the iron stores they developed in the womb start to deplete. It's a perfect time to start supplementing with iron-rich foods.
Six months is also a time when their GI system has matured, they are receptive to new tastes and textures, and they're probably eyeing the food you’re eating.
This week the FDA released a draft proposal that would reduce the legal limits of allowed lead in processed foods for babies and children under the age of 2. While these trace leads are likely nothing to worry about, if learning this has made you consider making your own homemade baby food, we want you to know that it's a lot easier to pull off than you think.
Should I make my own homemade baby food?
Credit: Getty Images / PeopleImages
You can potentially save a lot of money by making baby food at home.
I'm an atypical proselytizer of baby food making: I loved my epidural and Enfamil was my best friend during my son's first year. The truth is, I found making my own baby food to be a whole lot easier than figuring out the baby food aisle. Plus, it was a whole lot cheaper.
Homemade organic baby food is estimated to be 45% less expensive than store-bought. Non-organic homemade baby food will save you even more.
It really doesn’t take any special equipment, know-how, or skills and it takes less culinary prowess than cooking for the adults in your life. Before you embark, here are a few things that are helpful to know.
Related content
How can I make homemade baby food?
You can more or less turn anything into a baby food purée for stage 1 eaters, and once they are nibbling at small bites it gets even easier. There are some rules to follow, however.
Keep things super clean
Babies have a weaker immune system than older children and adults. Be sure to up your cleaning game before prepping baby food. Only prepare foods on a very clean surface and with freshly cleaned equipment. Now is the time to sing the Happy Birthday song two full times when you wash your hands.
Be sure to up your cleaning game before prepping baby food. Only prepare foods on a very clean surface and with freshly cleaned equipment. Now is the time to sing the Happy Birthday song two full times when you wash your hands.
Be wise about diluting
Since babies can’t drink cow’s milk until they are 1 year old, don’t use it in your homemade baby food. Breast milk, formula, or plain filtered water are better choices to thin out purées. Keep in mind that too much liquid makes for runny food, so add a little bit at a time.
You can also add a few teaspoons of olive oil for a little bit of flavor and to help with constipation. If you’re tempted to use coconut oil it’s important to know that it should be added with caution as it could potentially reveal a sulfate allergy.
Keep allergies in mind
Before introducing your child to any foods with typical allergens, including dairy products, eggs, and nuts, consult with your child’s pediatrician.
Hold the honey
Honey and maple syrup are off-limits for babies under 12 months of age.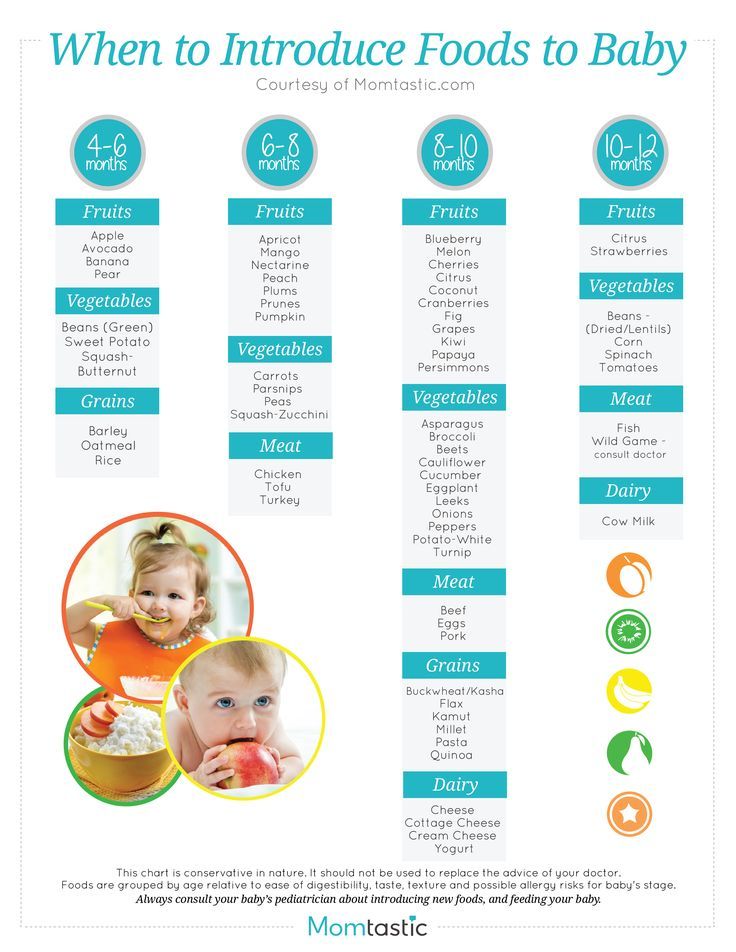 Not only can these ingredients cause issues with glucose levels, but there can also be small traces of botulism in each of these ingredients. Don’t give them to your baby until they are 1 year old, but it's recommended you wait until age 2 before giving sweeteners of any kind.
Not only can these ingredients cause issues with glucose levels, but there can also be small traces of botulism in each of these ingredients. Don’t give them to your baby until they are 1 year old, but it's recommended you wait until age 2 before giving sweeteners of any kind.
While you’re at it, also hold the salt and be sure to only use mild spices, like basil, oregano, thyme, coriander, cinnamon, nutmeg, turmeric, cumin, fennel, and dill in your baby’s food.
When can I give my baby solids?
Credit: Getty Images / stock_colors
Most fruits are a good entry point to solid foods.
The Centers for Disease Control and the American Academy of Pediatrics say that when children are 6 months of age they can be introduced to foods other than breast milk or infant formula. “Solids” refers to any food that isn’t breast milk or baby formula. The purées you buy or will make at home are considered first solids for babies.
Is my baby ready for solids?
If your baby has good head and neck control, is able to sit up, and is showing an interest in food by leaning forward and/or opening their mouth when food is near, they are likely ready to start eating solids. If you're not sure if they are truly ready, you should double-check with your pediatrician.
If you're not sure if they are truly ready, you should double-check with your pediatrician.
What foods should I Introduce first?
While mashed bananas and avocados are typical first foods, the American Academy of Pediatrics says that most children do not need to be introduced to foods in a certain order, though there are some foods that are easier for little bellies to manage than others.
We highly recommend the first foods database by Solid Starts. It breaks down every food, the earliest age you can introduce it to babies, common allergens, and methods for safe preparation.
Visit the Solid Starts database
What do I need to make homemade baby food?
Credit: Reviewed / Betsey Goldwasser / Jackson Ruckar
A baby food maker is fun to use and helps with portion control, but you can also opt for an immersion blender or a Vitamix.
You really don’t need a lot and you probably already have what you need in your kitchen.
If you’d like to investigate a baby food maker, my family felt like they made things more manageable, they made cleanup easier, and they made baby food-making a bit more fun.
We had the baby Nutribullet Baby and loved it. It was easy to use, easy to clean, and it allowed me to quickly blend up small, unseasoned parts of meals I was making for myself. I never had to worry about storage or spoilage and it took up very little space on my counter.
$66 at Babylist
Parenting editor, Anna Lane, had the Beaba Babycook and says she loved how it steamed and puréed all in one.
$160 at Babylist
If you don’t want to go the baby food maker route, you can definitely use household items you probably already have, including a blender that can blend through tough, fibrous foods, like broccoli, carrots, or beets.
Here is a list of what you’ll need:
- A hand-held immersion blender
- A Vitamix (These are best if you want to also make your own rice cereals)
- A sieve or strainer (to remove seeds and larger bits of food)
- A pot and steamer basket to steam vegetables, fruits, and meats.

How to store homemade baby food
Credit: Getty Images / Magone
Once prepared, homemade purées can be kept in the refrigerator for one to two days.
First, be sure to only serve your baby a couple of tablespoons of purées at a time. Once a spoon has gone into the food, it essentially has contaminated it, making it unsuitable to be saved for later (this goes for store-bought baby food, too).
Once prepared, homemade purées can be kept in the refrigerator for one to two days or in the freezer for up to two months. Try spooning them into single-serving containers or freezing them in ice cube trays for small, easy-to-serve portions. We recommend covered ice cube trays (to keep it sanitary) that are made from silicon, so it's easier to pop portions out one by one.
$20 at Amazon
Heat up leftovers by warming them on the stove or in the microwave and then let them cool until room temperature. Never serve a baby hot food.
For microwaved foods, make sure to thoroughly stir and check that there aren’t any hot spots.
There you go! Easier than getting dressed and making a trip to the grocery store. And if all of this is too much, there is always baby-led weaning!
The product experts at Reviewed have all your shopping needs covered. Follow Reviewed on Facebook, Twitter, Instagram, TikTok or Flipboard for the latest deals, product reviews and more.
Prices were accurate at the time this article was published but may change over time.
Closer to Zero: Reducing Childhood Exposure to Contaminants from Foods
About | Approach | Action Items & Proposed Timeline | On-Going Work | Announcements
About Closer to Zero
The FDA’s goal is to reduce dietary exposure to contaminants to as low as possible, while maintaining access to nutritious foods. The agency’s work to date has resulted in significant progress in reducing exposure to environmental contaminants from foods and Closer to Zero builds on this progress. We have prioritized foods commonly eaten by babies and young children because their smaller body sizes and metabolism make them more vulnerable to the harmful effects of these contaminants. To meet the goals of Closer to Zero, we have focused our efforts on:
We have prioritized foods commonly eaten by babies and young children because their smaller body sizes and metabolism make them more vulnerable to the harmful effects of these contaminants. To meet the goals of Closer to Zero, we have focused our efforts on:
Research and Analysis:
- developing new and improved testing methods to measure lower levels of contaminants in food;
- conducting surveys on foods commonly eaten by infants and young children to understand variability in concentrations of arsenic, lead, cadmium, and mercury in these foods;
- working with federal partners to identify strategies to reduce contaminant levels in food products;
- evaluating consumption patterns for different populations;
- measuring dietary exposures to these contaminants;
- identifying reference levels for these contaminants to determine levels of concern; and
- understanding how nutrients can help protect against the health effects associated with contaminants.

Regulation:
- establishing action levels;
- increasing targeted compliance and enforcement activities; and
- monitoring levels over time to determine potential adjustments to proposed action levels.
Consultation:
- encouraging adoption of agricultural and processing best practices by industry to lower levels of environmental contaminants in agricultural commodities and products.
Reducing levels of contaminants in foods is complicated and multifaceted. It is crucial to ensure that measures taken to limit arsenic, lead, cadmium, and mercury in foods do not have unintended consequences—like eliminating from the marketplace foods that have significant nutritional benefits or reducing the presence of one element while increasing another.
The FDA is committed to a science-driven, transparent, and inclusive process that will include active stakeholder engagement and public sharing of data and information. We will update our action items and timelines as new data, information, and resources become available.
We will update our action items and timelines as new data, information, and resources become available.
More information, including links to FDA guidance and data tables, can be found on FDA’s lead, arsenic, mercury, and testing results webpages.
Understanding the FDA’s Approach
Closer to Zero uses a science-based, iterative approach for achieving continual improvements over time.
The Four Stages of the FDA’s Approach:
Evaluate the scientific basis for action levels. The cycle of continual improvement starts with the FDA evaluating existing data from routine testing of the food supply, research and data on chemical analytical methods, toxicological assays, exposure and risk assessments, and other relevant scientific information. Through a process that may include engagement with stakeholders, advisory committees, public workshops, and consultation with scientific experts, federal agency partners and other stakeholders, the agency will establish interim reference levels (IRLs) as appropriate. An IRL is a measure of exposure to a contaminant from food that the FDA may use to determine if the amount of exposure to the contaminant across foods could result in a specific health impact.
An IRL is a measure of exposure to a contaminant from food that the FDA may use to determine if the amount of exposure to the contaminant across foods could result in a specific health impact.
Propose action levels. The IRLs may be among the key factors that inform the development of the FDA’s proposed action levels for arsenic, lead, cadmium, and mercury in categories of baby foods (e.g., cereals, pureed fruits and vegetables) and other foods commonly eaten by babies and young children. After the FDA identifies action levels, the agency submits our draft guidance on action levels for review by other federal agencies, including The White House.
Consult with stakeholders on proposed action levels, including the achievability of action levels. For each individual element in every identified category of food, the FDA will gather data and other information through a process of consultation which could include workshops, scientific meetings, and collaboration with federal partners to assess, among other things, the achievability and feasibility of the proposed action levels and the timeframes for reaching them.
Finalize action levels. The FDA will use the information gathered from stakeholders, updated scientific research, and routine monitoring of data to make any needed adjustments and finalize action levels.
The FDA's Approach in Action
Once the FDA has published final action levels, the agency will establish a timeframe for assessing industry’s progress toward meeting the action levels and resume the cycle to determine if the scientific data support efforts to further adjust the action levels.
The availability of data and additional research needs for arsenic, lead, cadmium, and mercury are different. Therefore, because of currently available data, we will start with proposing action levels for lead while we evaluate data for arsenic, cadmium, and mercury. We will then progress through the cycle for each element as we gather more data and information across various categories of food eaten by babies and young children.
As action levels are finalized, we will continue the cycle of continual improvement, addressing arsenic, lead, cadmium, and mercury to evaluate whether downward adjustments of interim reference levels should be made; proposing new action levels, as appropriate; consulting with stakeholders on feasibility, achievability, and other issues; and adjusting (as needed) and finalizing action levels.
While action levels are a useful tool to drive down levels of contaminants in foods, the agency does not need an action level for a contaminant to take enforcement action on a food product that violates safety laws. By law food manufacturers and processors have a responsibility to implement preventive controls as needed to significantly minimize or prevent exposure to chemical hazards—including lead, arsenic, cadmium, and mercury. If the agency finds that the level of a contaminant in a food causes the food to be unsafe, we take action, which may include working with the manufacturer to resolve the issue and taking steps to prevent the product from entering, or remaining in, the U.S. market.
Planned Action Items
Action Levels
| Contaminant/Commodity | Evaluate the Gather data and collaborate with federal partners. | Propose Draft Action Levels Propose action levels for specific contaminants for foods or food groups in draft guidance to industry and open for public comment. | Consult with Stakeholders Engage with stakeholders to assess, among other things, feasibility and best practices. | Finalize Action Review new scientific data, assess progress on reducing environmental contaminants in products and feasibility of attaining even lower levels. |
|---|---|---|---|---|
| Lead in Juices | February 2020: Interim Reference Level for lead identified | April 2022: Issued draft guidance | June 2022: Stakeholder webinar | No update |
| Lead in Foods Intended for Babies and Young Children | August 2022: Interim Reference Level for lead updated | April 2022: Developed action levels and submitted draft guidance for interagency review January 2023: Issued Draft Guidance | March 2023: Stakeholder webinar | No update |
| Arsenic in Foods Intended for Babies and Young Children | 2022-2023: Continue evaluating, together with federal partners, the science for arsenic and other work related to establishing interim reference levels. | 2024: Develop action levels and submit draft guidance for interagency review | No update | No update |
| Cadmium in Foods Intended for Babies and Young Children | 2022-2023: Continue evaluating the foundational science for cadmium to establish interim reference levels | 2024: Develop action levels and submit draft guidance for interagency review | No update | No update |
| Mercury in Foods Intended for Babies and Young Children | 2022-2023: FDA and federal partner’s sponsor study on the role of seafood consumption in child growth and development | January 2023: NASEM webcast | No update | No update |
Research, Monitoring, and Compliance
| Activities | Status |
|---|---|
| Research: Cadmium - toxicology | 2022: Completed systematic review of adverse health effects from oral cadmium exposure; review of adverse health effects of cadmium in infants and children, modeling of low bone mass and osteoporosis in US adults; and modeling of cadmium dose response. |
| Research: Lead - toxicology | 2020: Identified Interim Reference Level for lead to calculate maximum daily intake from food. 2022: Completed updating the Interim Reference Level for lead to align with CDC’s 2021 updated blood lead reference value (CDC’s BLRV of 3.5 µg/dL). |
| Research: Arsenic - toxicology | Ongoing: evaluating data and other information on the dose response of arsenic. |
| Research: Mercury – toxicology | 2022-2023: Contracted NASEM for an independent consensus study on the role of seafood in child growth and developing, including mercury. |
| Research: Predictive toxicology | 2022: Applying predictive toxicology to assess toxicity of arsenic, lead, cadmium, and mercury using predictive toxicology. FDA assessed developmental toxicity of arsenic and mercury in a non-vertebrate, alternative model using C. elegans and developmental toxicity and neurotoxicity of arsenic in a vertebrate alternative model using zebrafish. elegans and developmental toxicity and neurotoxicity of arsenic in a vertebrate alternative model using zebrafish. |
| Research: Methods development | Ongoing: Developing and validating analytical methods for toxic elements in foods. |
| Research, Monitoring, and Compliance: Testing foods commonly consumed by babies and young children, for arsenic, lead, cadmium and mercury. | 2022: FDA Total Diet Study (TDS) FY2018-FY2020 Elements Report and Data 2022: A survey of toxic elements in ready to eat baby foods in the US market 2021 2022: Analytical Results for Lead in Juice -TEP FY2005-FY2018 - PDF, in XLSX 2022: Analytical Results for Total Arsenic in Single-Strength Apple Juice-TEP (FY2013-FY2022) 2022: Speciation Results from Arsenic Analysis in Single-Strength Apple Juice -TEP (FY2013-FY2022) |
| Issue guidance chapter on chemical hazards in the Draft Guidance for Industry on Hazard Analysis and Risk-Based Preventive Controls for Human Food | Target 2024 |
| Research: Consumer studies to inform educational content for target audiences | Ongoing: In-depth interviews with health educators and additional focus groups with parents and caregivers are under development. Ongoing: Focus groups conducted with parents and caregivers. Ongoing: FDA and USDA collaboration to gather informational needs for consumer and health professional education. |
Public Meetings, Workshops, and Webinars*
| Events | Related Links |
|---|---|
| Stakeholder Webinar on Action Levels for Lead in Food Intended for Babies and Young Children: Draft Guidance for Industry March 2, 2023 | Event Page and Recording |
| Partnership for Food Safety Education 2023 Consumer Food Safety Education Conference, Pre-conference Workshop: FDA’s Closer to Zero Action Plan – What’s the Food Safety Message? March 1, 2023 | Event Page |
| The Role of Seafood Consumption in Child Growth and Development (Meeting #2), February 23, 2023 | Recording |
| NIH-FDA Joint Scientific Workshop “Bridging the Biological and Communication Sciences on Nutrients and Environmental Contaminants in Foods to Support Child Development” February 9-10, 2023 | Event Page Recording Day 1 Recording Day 2 |
| National Academies of Sciences, Engineering, and Medicine Committee Review of The Role of Seafood in Child Growth and Development Information Gathering Session, January 19, 2023 | Recording |
| Science Board to the Food and Drug Administration Advisory Committee – Cadmium, December 8, 2022 | Event Page and Recording |
| FDA Stakeholder Webinar on the Draft Guidance for Industry on Action Levels for Lead in Juice, June 14, 2022 | Event Page and Recording |
| JIFSAN-CFS3 Advisory Council Virtual Annual Symposium: Understanding of the Impact of Arsenic, Cadmium, and Lead Across the Food Supply, May 25-26, 2022 | Event Page Recording link |
| USDA-FDA Joint Public Meeting - Closer to Zero: Impacts of Toxic Element Exposure and Nutrition in the Food System, April 27, 2022 | USDA Closer to Zero Page |
| USDA NIFA Workshop on Toxic Elements in Food: Identification of Critical Knowledge Gaps to Ensure a Safe Food Supply, April 4-5, 2022 | Workshop report |
| FDA Grand Rounds on Closer to Zero, December 9, 2021 | Event Page and Recording |
| FDA-Society of Toxicology Colloquia on Emerging Toxicological Science: Challenges in Food and Ingredient Safety - Arsenic and Children’s Health, December 1, 2021 | Event page |
| FDA Public Meeting: Closer to Zero Action Plan: Impacts of Toxic Element Exposure and Nutrition at Different Crucial Developmental Stages, on Closer to Zero, November 18, 2021 | Event page and Recording |
*Includes public events that are hosted, co-hosted, and/or funded/sponsored by FDA.
Ongoing Work to Support Closer to Zero
Trends in Exposure to Toxic Elements from Foods for Babies and Young Children (PDF: 1.7MB)
To help us reach the Closer to Zero goals and build on the significant progress that has already been made in reducing contaminants in the food supply, we will also continue:
- Developing and validating analytical methods.
- Continuing toxicological research on impacts of arsenic, lead, cadmium, and on development in children.
- Developing new dose-response models to consider the probability of other adverse health effects in different sub-populations, including infants and young children.
- Collaborating with the U.S. Department of Agriculture on research on agronomic techniques that may mitigate uptake of these elements in agricultural commodities
- Collaborating with the National Institutes of Health and the Centers for Disease Control and Prevention to better understand impacts of these elements on development and the role of nutrition for mitigating those impacts.

- Evaluating potential impact of new technologies, interventions, or mitigation controls to reduce exposure and resulting risk to consumer health.
- Reevaluating risk assessments based on declining levels of toxic elements in foods.
The FDA’s regulatory actions, along with research and collaboration with all of our stakeholders – industry, advocacy, policy makers, academia, and consumers – will result in significant reductions in exposures to arsenic, lead, mercury, and cadmium, among children and the general population and have lasting public health impact.
For FDA’s published research, please visit our FDA Monitoring and Testing sections on the Lead, Arsenic, Cadmium, and Mercury pages.
Announcements
- FDA to Hold Webinar on the Draft Guidance for Industry on Action Levels for Lead in Food Intended for Babies and Young Children (January 26, 2023)
- FDA Issues Guidance for Industry on Action Levels for Lead in Baby Foods (January 24, 2023)
- Registration Open for Scientific Workshop Exploring Research on Nutrients and Environmental Contaminants in Foods and Child Development (January 12, 2023)
- Update on Study of the Role of Seafood Consumption in Child Growth and Development (January 12, 2023)
- FDA and Federal Partners Launch Study on the Role of Seafood Consumption in Child Growth and Development (October 11, 2022)
- FDA to Hold Webinar on the Draft Guidance for Industry on Action Levels for Lead in Juice (June 20, 2022)
- FDA Issues Draft Guidance to Industry on Action Levels for Lead in Juice (April 27, 2022)
- FDA Announces First Closer to Zero Action Plan Public Meeting (October 8, 2021)
- FDA Statement: FDA Releases Action Plan for Reducing Exposure to Toxic Elements from Foods for Babies, Young Children (April 8, 2021)
- FDA Shares Action Plan for Reducing Exposure to Toxic Elements from Foods for Babies and Young Children (April 8, 2021)
- FDA Letter to Industry on Chemical Hazards, including Toxic Elements, in Food and Update on FDA Efforts to Increase the Safety of Foods for Babies and Young Children (March 5, 2021)
- FDA Statement: FDA Announces New Actions Aimed at Further Reducing Toxic Elements in Food for Babies, Young Children (March 5, 2021)
- FDA Response to Questions About Levels of Toxic Elements in Baby Food, Following Congressional Report (February 16, 2021)
VIEW / American babies deprived of baby food :: In the world
America plunged into an unprecedented crisis for her, affecting the most defenseless members of society - children. Millions of little Americans were left without specialized food; baby food is almost impossible to find in stores. New York has even declared a state of emergency because of this. What are the reasons for what is happening and why do American politicians remember about Ukraine because of this?
Millions of little Americans were left without specialized food; baby food is almost impossible to find in stores. New York has even declared a state of emergency because of this. What are the reasons for what is happening and why do American politicians remember about Ukraine because of this?
Just a few years ago, such news could only appear in the pages of a fantastic literary work or in the script of a disaster movie. But the rapidly changing world has accustomed everyone to the fact that the most impossible stories become reality. A few days ago, the mayor of New York, one of the largest cities on our planet with a population of over 18 million, signed a decree declaring a state of emergency on its territory.
The reason for this decision sounds shocking - the lack of baby food in the city. An even bigger shock for Americans for months now is that the lack of baby food is acutely felt throughout the United States. Americans are horrified to realize that their youngest fellow citizens lack the food they need to survive.
"The nationwide shortage of infant formula is causing unimaginable pain and anxiety to families across New York City, and we must act now," Mayor Adams said in a message to the city's residents. “The emergency order will give us the opportunity to crack down on any retailer who wants to capitalize on this crisis by driving up the price of this product. Our message to mothers and families struggling is simple: our city will do everything in its power to help you through this difficult time.”
Fighting speculators under the introduced state of emergency, the authorities are threatening to act harshly. New York City Department of Consumer and Worker Welfare (NYDCWP) Commissioner Vilda Vera Mayuga is urging residents who notice significant increases in the price of, or illegal sales of, infant formula to immediately report them to the special short number 311. Explained that "overpriced" means a price that is 10% or more higher than what a resident of the city could have purchased a similar product in the period from 30 to 60 days before the declaration of a state of emergency. What measures will be taken against unscrupulous sellers, the department does not report.
What measures will be taken against unscrupulous sellers, the department does not report.
New York was the first city in which the situation with a vital product required the introduction of such drastic measures as a state of emergency. However, the situation in the city and the state of the same name is not unique to America today - infant formula is not available in almost the entire country, having previously jumped in price by 40% by early April.
The shortage of baby food stocks that began in 2021 has reached critical levels this year in the states of Iowa, North and South Dakota, and Missouri. In Tennessee and Texas, out-of-stock rates are already in excess of 54% and continue to skyrocket thanks to the growing panic in the country. In another 26 states, more than 50% of stocks are already missing, while back in January 2022 out-of-stock was 23%.
But in February thunder struck. Abbott Laboratories, the world's largest baby food manufacturer, has suspended production and recalled a huge batch of infant formulas produced by it, known throughout the world Similac, because of the pathogenic bacteria found in them that are dangerous to children's health.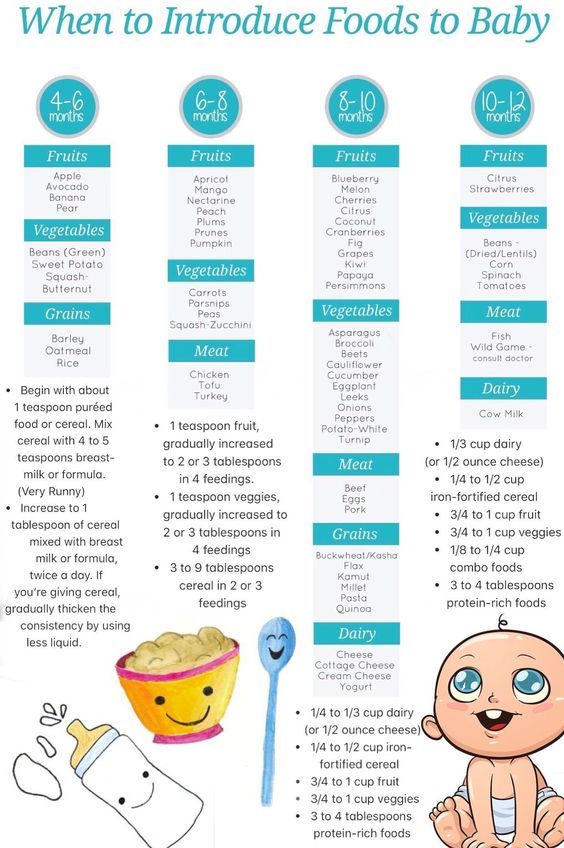 The scandal took on such serious proportions after several children were hospitalized after drinking Abbott formulas. An investigation is currently underway into whether the deaths of at least two babies were related to the use of Abbott products.
The scandal took on such serious proportions after several children were hospitalized after drinking Abbott formulas. An investigation is currently underway into whether the deaths of at least two babies were related to the use of Abbott products.
It should be noted that the baby food market in the US is problematic due to its steady decline. According to US Census Bureau estimates released in March this year, in half of all states and almost three-quarters of all counties between July 1, 2020 and June 30, 2021, deaths exceeded births. Studying statistics with a longer time period of coverage is also disappointing for Americans - since the mid-2000s, the number of newborns has been declining every year, with the exception of a short-term surge in 2014.
At the same time, the US baby food market is virtually monopolized by several large manufacturers. Abbott, founded back in 1888, has been among the industry leaders for many years. Summing up the past year, Abbott Laboratories CEO Robert Ford called it "an outstanding year for the company. " Indeed, the company's profits rose steadily, with a net income reported in the final report to investors of $7.07 billion.
" Indeed, the company's profits rose steadily, with a net income reported in the final report to investors of $7.07 billion.
With about 40% market share, along with Mead Johnson and Nestle, the company participates in the government's nationwide WIC program for women, babies and children. This program provides supplemental nutrition for expecting mothers, women who are breastfeeding from low-income families, and children under the age of five. It is also under this program that about half of all infant formula in the United States is sold.
The cessation of production of mixtures under the Abbott brand, therefore, did not hit the investors in the first place, who managed to share considerable profits. First of all, the most vulnerable categories of the population of the states - women, children and low-income families - became victims of the acute shortage of baby formula. American doctors are sounding the alarm - the number of food poisoning in infants is growing rapidly, as parents from poor families are trying to make substitutes for mixtures from dairy products in artisanal conditions. Obviously, no one controls the shelf life, sterility, and the very safety of the ingredients used for the digestion of children.
Obviously, no one controls the shelf life, sterility, and the very safety of the ingredients used for the digestion of children.
Parents facing the dreaded prospect of infant hunger are trying by all means to find an alternative to a product that is disappearing from the shelves. Within a few weeks, the flow of Americans back to Mexico was established - in this neighboring country of the United States, baby food has not yet become a shortage. Due to logistical and customs difficulties, it is not necessary to count on mass deliveries of the "formula" from Europe. There is no certainty that the EU countries, which continue to “shoot themselves in the foot” with the sanctions war, will be happy to share strategic reserves.
It is not known how long the FDA (US Food & Drug Administration) - the US federal agency responsible for product safety control in the sale of food and drugs - will deal with the elimination of Abbott's production problems: according to some forecasts, this will not happen before July. At the same time, realizing the scale of the crisis and the need to increase the supply of mixtures to the United States from abroad, the agency announced that until November 14 it will temporarily change its extremely strict rules and will consider the possibility of importing products of each manufacturer individually, at its discretion.
At the same time, realizing the scale of the crisis and the need to increase the supply of mixtures to the United States from abroad, the agency announced that until November 14 it will temporarily change its extremely strict rules and will consider the possibility of importing products of each manufacturer individually, at its discretion.
The Biden administration, extremely concerned about the proxy war with Russia and the process of creating new alliances against China, by all indications, does not yet fully realize the scale of the crisis that could erupt in connection with the current situation.
The steps taken so far have not brought a positive result, but rather, on the contrary, have caused even greater irritation of the Americans. Several hundred containers delivered by US military aircraft from the German Ramstein base, another strategic operation "Flying Baby Food" to deliver children's "formula" can only temporarily moderate the panic of parents and plug gaps in the food collapse.
The strategic solution to the problem lies on a different plane. The few congressmen who opposed the allocation of huge funds under the so-called Lend-Lease Act in defense of democracy in Ukraine also appealed to the US president to finally pay attention to him.
Georgia Republican Party member Marjorie Taylor Greene, voting against this adventurous project in Congress, told her colleagues and the president, “We have $40 billion, but no baby food for American mothers and babies. There is an unknown amount of money for CIA activities in Ukraine, but no food for American babies and mothers. There are $54 million in spending to fight COVID-19for Ukraine, but no food for American babies and mothers. There is $900 million for nonprofits in Ukraine, but no food for American babies and mothers. There is $8.7 billion in economic support and funding for Ukraine, but no food for American mothers and babies… The American people do not support paying for the constant US interference in the foreign affairs of other countries while our own government is ruining our country. ”
”
Will the voices of hundreds of thousands of American women and mothers be heard if the threat of child hunger ceases to be an emergency within the boundaries of New York alone?
Watch more videos on the YouTube channel VZGLYAD
Hungry children of a superpower: the United States is facing a baby food crisis
There is not enough food for the smallest in the country - the supply of infant formula has almost halved. Parents are encouraged to temporarily use cow's milk to feed their babies, but if possible, not to do so for a long time. Mothers dilute mixtures, feed their children less, spend several hours a day looking for food for their babies. Urgent flights of military aircraft deliver shipments of baby food from abroad by order of the President.
Empty shelves for baby food in a New Jersey store, USA
© Tayfun Coskun/Anadolu Agency via Getty Images
All of this is not a reality in any third world country, but the daily life of the last weeks of many families in the United States. The causes of the crisis are exclusively internal - monopoly in the economy, plus excessive, but, as it turned out, ineffective state regulation, as well as protectionism.
The causes of the crisis are exclusively internal - monopoly in the economy, plus excessive, but, as it turned out, ineffective state regulation, as well as protectionism.
A real American taste
In February, when the US administration began to mobilize support for Ukraine from Western countries, as well as increasing sanctions pressure on Russia, the news about the shutdown of the plant for the production of Abbott infant formula in the town of Sturgis, Michigan, passed almost unnoticed. Only about 10 thousand people live in Sturgis, located between Chicago and Detroit, and hardly anyone would remember this town if it were not for the plant. Abbott controls about 42% of the US infant formula market, and half of its products are made at this facility - the largest in the country in this area.
The story began with the fact that four children were poisoned by mixtures from the plant, two of them fatally, and the enterprise temporarily stopped working. So the market lost about 20% of the supply.
Read also
Bread or gadgets: what is going to replace globalization?
The situation was aggravated by other circumstances closely related to the peculiarities of the economic and state structure of the United States. The Federal Food and Drug Administration (FDA) could not agree on the opening of the enterprise for three months. And prohibitive trade tariffs, which were introduced to support American companies like Abbott, prevented exports from replacing their missing products - Abbott and three other similar corporations together control 90% of the US infant formula market. Another factor was that current social assistance programs in a number of states provide benefits to needy families, by virtue of contracts in force with Abbott, only when purchasing products from this manufacturer.
It is also worth noting that breastfeeding is not common in the US, as there is no paid parental leave. And measures to promote breastfeeding in the workplace have had limited impact and are more of a symbol of corporate progressiveness than widespread support for mothers, especially when it comes to low-paying jobs.
An Invisible Crisis
The baby food crisis lingered for three months before hitting the front pages of the newspapers and becoming a topic of discussion in Congress (while, for example, Ukraine's legislature had already approved several billion military aid). Parents bought up all the available stocks and in a matter of hours they are sorting out new batches. Stores were forced to impose restrictions on the volume of sales of mixtures in one hand, but the shelves are still empty.
If there was a 2-8% monthly mix shortage at the Sturgis plant closure due to supply chain disruptions due to the COVID-19 pandemic, then in April this figure rose to 30%, by May 8 - up to 43%, by May 15 - 45%.
Read also
The head of the IMF called on countries to lower trade barriers to combat rising prices
The crisis developed, but did not attract much attention from the middle and rich sections of society, until the deficit grew enough to affect everyone. In large cities with a large number of retailers, which close numerous supply chains from different manufacturers, it is still possible to find the right product, although this may take some time, and salaries are usually higher there. The situation is much worse in small towns or ethnic enclaves, where there is often one large supermarket for thousands of inhabitants, which has contracts with a limited number of suppliers (for example, this was the Tops supermarket in Buffalo, where a white supremacist shot ten people on May 14) . The hardest hit are the poor, members of racial and ethnic minorities, living in disadvantaged areas with poorly developed infrastructure and dependent on federal or municipal social programs.
The situation is much worse in small towns or ethnic enclaves, where there is often one large supermarket for thousands of inhabitants, which has contracts with a limited number of suppliers (for example, this was the Tops supermarket in Buffalo, where a white supremacist shot ten people on May 14) . The hardest hit are the poor, members of racial and ethnic minorities, living in disadvantaged areas with poorly developed infrastructure and dependent on federal or municipal social programs.
In recent days, there has been another explanation why the crisis has been left unaddressed for a long time - despite all the talk in the United States about gender equality, gender roles and distribution of responsibilities, it is women who feed the children, and it is mostly men who make decisions and occupy leadership positions. In the current composition of the US Congress, only 144 of 539 seats (27%) are held by women - this is the maximum number in the history of the American legislature.
Who is to blame and what to do
Only last week, the federal authorities noticed the growing crisis. The White House ordered to organize an urgent delivery of infant formula from abroad, involving military aviation in this. Thousands of tons of baby food have already arrived in the United States over the weekend from the Ramstein base in Germany. Families in need who cannot afford subsidized formula were promised new benefits. The FDA has received an additional $28 million to improve the way it inspects and approves US and foreign products. The FDA has already agreed to reopen the Sturgis plant from this Monday, although it could be weeks before new formulas hit the shelves. US President Joe Biden activated the Defense Production Act of 1950, which allows the federal government to directly intervene in the production and distribution of products. Such measures have already been practiced in the event of shortages in the past.
© Jon Cherry/Getty Images
The search for the guilty began. The Speaker of the House of Representatives, the leader of the Democrats in Congress, Nancy Pelosi, threatened Abbott with criminal prosecution - after all, it was precisely because of their problems in production that the plant stopped. However, these are only assumptions - at a congressional hearing, an FDA representative said that the investigation into the incident with the poisoning of babies has not yet been completed. He also did not answer about the reasons for the three-month-long inspection of the plant, again referring to the secrecy of the investigation.
The Speaker of the House of Representatives, the leader of the Democrats in Congress, Nancy Pelosi, threatened Abbott with criminal prosecution - after all, it was precisely because of their problems in production that the plant stopped. However, these are only assumptions - at a congressional hearing, an FDA representative said that the investigation into the incident with the poisoning of babies has not yet been completed. He also did not answer about the reasons for the three-month-long inspection of the plant, again referring to the secrecy of the investigation.
Read also
How Americans think: what's on Biden's mind and tongue
Republicans traditionally blame Democrats: federal regulator failed in mission; democrats, boasting of being close to the people, cannot feed children in poor families. In fact, this new crisis is the very last thing the Democratic Party needs ahead of the midterm elections this fall. She responds with accusations against large corporations like Abbott that have destroyed competition, are trying to interfere with government regulation, and also seek tax breaks at the expense of voters.